Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices
- PMID: 9705999
- PMCID: PMC2231061
- DOI: 10.1111/j.1469-7793.1998.499bk.x
Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices
Abstract
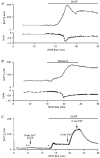
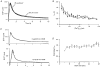
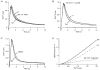
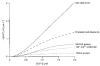
1. The mechanisms governing the return of intracellular calcium (Cai2+) to baseline levels following depolarization-evoked [Ca2+]i rises were investigated in Purkinje cell somata using tight-seal whole-cell recordings and fura-2 microfluorometry, for peak [Ca2+]i ranging from 50 nm to 2 microM. 2. Cai2+ decay was well fitted by a double exponential with time constants of O.6 and 3 s. Both time constants were independent of peak [Ca2+]i but the contribution of the faster component increased with [Ca2+]i. 3. Thapsigargin (10 microM) and cyclopiazonic acid (50 microM) prolonged Cai2+ decay indicating that sarco-endoplasmic reticulum Ca2+ (SERCA) pumps contribute to Purkinje cell Cai2+ clearance. 4. A modest participation in clearance was found for the plasma membrane Ca2+ (PMCA) pumps using 5,6-succinimidyl carboxyeosin (40 microM). 5. The Na(+)-Ca2+ exchanger also contributed to the clearance process, since replacement of extracellular Na+ by Li+ slowed Cai2+ decay. 6. Carbonyl cyanide m-chlorophenylhydrazone (CCCP, 2 microM) and rotenone (10 microM) increased [Ca2+]i and elicited large inward currents at -60 mV. Both effects were also obtained with CCCP in the absence of external Ca2+, suggesting that mitochondrial Ca2+ uptake uncouplers release Ca2+ from intracellular stores and may alter the membrane permeability to Ca2+. These effects were irreversible and impeded tests on the role of mitochondria in Cai2+ clearance. 7. The relative contribution of the clearance systems characterized in this study varied as a function of [Ca2+]i. At 0.5 microM Cai2+, SERCA pumps and the Na(+)-Ca2+ exchanger contribute equally to removal and account for 78% of the process. Only 45% of the removal at 2 microM Cai2+ can be explained by these systems. In this high [Ca2+]i range the major contribution is that of SERCA pumps (21%) and of the Na(+)-Ca2+ exchanger (18%), whereas the contribution of PMCA pumps is only 6%.
Figures



Similar articles
-
Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase.J Physiol. 2000 May 1;524 Pt 3(Pt 3):701-13. doi: 10.1111/j.1469-7793.2000.00701.x. J Physiol. 2000. PMID: 10790152 Free PMC article.
-
Calcium homeostasis in trigeminal ganglion cell bodies.Cell Calcium. 2007 Apr;41(4):389-96. doi: 10.1016/j.ceca.2006.08.014. Epub 2006 Oct 13. Cell Calcium. 2007. PMID: 17046058
-
Ca2+ clearance mechanisms in isolated rat adrenal chromaffin cells.J Physiol. 1996 Apr 15;492 ( Pt 2)(Pt 2):329-46. doi: 10.1113/jphysiol.1996.sp021312. J Physiol. 1996. PMID: 9019533 Free PMC article.
-
Multiple/heterogeneous Ca2+ stores in cerebellum Purkinje neurons.Comp Biochem Physiol Comp Physiol. 1993 Jun;105(2):205-11. doi: 10.1016/0300-9629(93)90196-b. Comp Biochem Physiol Comp Physiol. 1993. PMID: 8101152 Review.
-
Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores.Pharmacol Ther. 1991;50(2):191-232. doi: 10.1016/0163-7258(91)90014-d. Pharmacol Ther. 1991. PMID: 1662401 Review.
Cited by
-
Functional integration of calcium regulatory mechanisms at Purkinje neuron synapses.Cerebellum. 2012 Sep;11(3):640-50. doi: 10.1007/s12311-010-0185-6. Cerebellum. 2012. PMID: 20596808 Free PMC article. Review.
-
Contribution of plasma membrane Ca ATPase to cerebellar synapse function.World J Biol Chem. 2010 May 26;1(5):95-102. doi: 10.4331/wjbc.v1.i5.95. World J Biol Chem. 2010. PMID: 21540995 Free PMC article.
-
Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons.J Neurosci. 1999 Jun 1;19(11):4325-36. doi: 10.1523/JNEUROSCI.19-11-04325.1999. J Neurosci. 1999. PMID: 10341236 Free PMC article.
-
Modeling Fast-scan Cyclic Voltammetry Data from Electrically Stimulated Dopamine Neurotransmission Data Using QNsim1.0.J Vis Exp. 2017 Jun 5;(124):55595. doi: 10.3791/55595. J Vis Exp. 2017. PMID: 28605373 Free PMC article.
-
Enhanced synaptic inhibition in the cerebellar cortex of the ataxic PMCA2(-/-) knockout mouse.Cerebellum. 2013 Oct;12(5):667-75. doi: 10.1007/s12311-013-0472-0. Cerebellum. 2013. PMID: 23568408
References
-
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proceedings of the National Academy of Sciences of the USA. 1997;94:1488–1493. 10.1073/pnas.94.4.1488. - DOI - PMC - PubMed
-
- Bassani RA, Bassani JW, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflügers Archiv. 1995;430:573–578. - PubMed
-
- Bleakman D, Roback JD, Wainer BH, Miller RJ, Harrison NL. Calcium homeostasis in rat septal neurons in tissue culture. Brain Research. 1993;600:257–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

