Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice
- PMID: 9706001
- PMCID: PMC2231051
- DOI: 10.1111/j.1469-7793.1998.527bk.x
Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice
Abstract
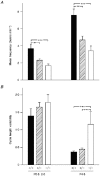
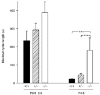
1. Molecular mechanisms underlying maturation of the central respiratory rhythm are largely unknown. Previously, we found that brain-derived neurotrophic factor (BDNF) is required for expression of normal breathing behaviour in newborn mice, raising the possibility that maturation of central respiratory output is dependent on BDNF. 2. Respiratory activity was recorded in vitro from cervical ventral roots (C1 or C4) using the isolated brainstem-spinal cord preparation from postnatal day (P) 0.5-2.0 and P4.5 wild-type mice and mice lacking functional bdnf alleles. 3. Loss of one or both bdnf alleles resulted in an approximately 50% depression of central respiratory frequency compared with wild-type controls. In addition, respiratory cycle length variability was 214% higher in bdnf null (bdnf-/-) animals compared with controls at P4.5. In contrast, respiratory burst duration was unaffected by bdnf gene mutation. 4. These derangements of central respiratory rhythm paralleled the ventilatory depression and irregular breathing characteristic of bdnf mutants in vivo, indicating that central deficits can largely account for the abnormalities in resting ventilation produced by genetic loss of BDNF. BDNF is thus the first growth factor identified that is required for normal development of the central respiratory rhythm, including the stabilization of central respiratory output that occurs after birth.
Figures
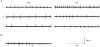
References
-
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. - PubMed
-
- Borday V, Kato F, Champagnat J. A ventral pontine pathway promotes rhythmic activity in the medulla of neonate mice. NeuroReport. 1997;8:3679–3683. - PubMed
-
- Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. - PubMed
-
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, Friedman B, Wiegand S, Vejsada R, Kato AC, DeChiara TM, Yancopoulos GD. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. - PubMed
-
- Dejours P. Chemoreflexes in breathing. Physiological Reviews. 1962;42:335–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

