Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries
- PMID: 9706005
- PMCID: PMC2231048
- DOI: 10.1111/j.1469-7793.1998.577bk.x
Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries
Abstract
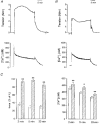
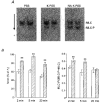
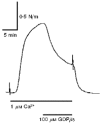
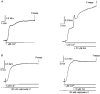
1. Mechanisms of Ca2+ sensitization of force production by noradrenaline were investigated by measuring contractile responses, intracellular Ca2+ concentration ([Ca2+]i) and phosphorylation of the myosin light chain (MLC) in intact and alpha-toxin-permeabilized rat mesenteric small arteries. 2. The effects of noradrenaline were investigated at constant membrane potential by comparing fully depolarized intact arteries in the absence and presence of noradrenaline. Contractile responses to K-PSS (125 mM K+) and NA-K-PSS (K-PSS + 10 microM noradrenaline) were titrated to 30 and 75%, respectively, of control force, by adjusting extracellular Ca2+ ([Ca2+]o). At both force levels, [Ca2+]i was substantially lower with NA-K-PSS than with K-PSS. With K-PSS, the proportion of MLC phosphorylated (approximately 30%) was similar at 30 and 75% of control force; with NA-K-PSS, MLC phosphorylation was greater at the higher force level (40 vs. 34%). 3. In alpha-toxin-permeabilized arteries, the force response to 1 microM Ca2+ was increased by 10 microM noradrenaline, and MLC phosphorylation was increased from 35 to 45%. The protein kinase C (PKC) inhibitor calphostin C (100 nM) abolished the noradrenaline-induced increase in MLC phosphorylation and contractile response, without affecting the contraction in response to Ca2+. Treatment with ATP gamma S in the presence of the MLC kinase inhibitor ML-9 increased the sensitivity to Ca2+ and abolished the response to noradrenaline. 4. The present results show that that in rat mesenteric small arteries noradrenaline-induced Ca2+ sensitization is associated with an increased proportion of phosphorylated MLC. The results are consistent with a decreased MLC phosphatase activity mediated through PKC. Furthermore, while MLC phosphorylation is a requirement for force production, the results show that other factors are also involved in force regulation.
Figures
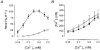
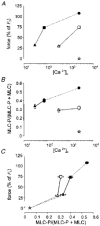
 , non-stimulated arteries (held in PSS) (n = 2); •, arteries stimulated for 5 min with NA-K-PSS, both with high [Ca2+]o (2·5 mM) (n = 2). Continuous lines connect paired data (n = 12), where the arteries were mounted on two double myographs run in parallel. Dashed lines indicate tentative curves connecting paired K-PSS or NA-K-PSS data with minimum and maximum values. Error bars indicate
, non-stimulated arteries (held in PSS) (n = 2); •, arteries stimulated for 5 min with NA-K-PSS, both with high [Ca2+]o (2·5 mM) (n = 2). Continuous lines connect paired data (n = 12), where the arteries were mounted on two double myographs run in parallel. Dashed lines indicate tentative curves connecting paired K-PSS or NA-K-PSS data with minimum and maximum values. Error bars indicate 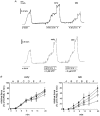
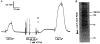
Similar articles
-
Calphostin C-sensitive enhancements of force by lysophosphatidylinositol and diacylglycerols in mesenteric arteries from the rat.Br J Pharmacol. 1996 Sep;119(1):15-22. doi: 10.1111/j.1476-5381.1996.tb15671.x. Br J Pharmacol. 1996. PMID: 8872351 Free PMC article.
-
Importance of inositol (1,4,5)-trisphosphate, intracellular Ca2+ release and myofilament Ca2+ sensitization in 5-hydroxytryptamine-evoked contraction of rabbit mesenteric artery.Br J Pharmacol. 1994 Feb;111(2):525-32. doi: 10.1111/j.1476-5381.1994.tb14769.x. Br J Pharmacol. 1994. PMID: 8004397 Free PMC article.
-
Increase by lysophosphatidylcholines of smooth muscle Ca2+ sensitivity in alpha-toxin-permeabilized small mesenteric artery from the rat.Br J Pharmacol. 1996 Mar;117(6):1238-44. doi: 10.1111/j.1476-5381.1996.tb16721.x. Br J Pharmacol. 1996. PMID: 8882621 Free PMC article.
-
Mechanisms of vasoconstriction induced by endothelin-1 in smooth muscle of rabbit mesenteric artery.J Physiol. 1994 Jun 1;477(Pt 2):253-65. doi: 10.1113/jphysiol.1994.sp020188. J Physiol. 1994. PMID: 7932217 Free PMC article.
-
A hypothesis for the mechanism of receptor and G-protein-dependent enhancement of vascular smooth muscle myofilament Ca2+ sensitivity.Can J Physiol Pharmacol. 1994 Nov;72(11):1420-6. doi: 10.1139/y94-205. Can J Physiol Pharmacol. 1994. PMID: 7767888 Review.
Cited by
-
The involvement of protein kinase C in myosin phosphorylation and force development in rat tail arterial smooth muscle.Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):573-82. Biochem J. 2000. PMID: 11085953 Free PMC article.
-
The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction.Curr Hypertens Rep. 2003 Feb;5(1):66-72. doi: 10.1007/s11906-003-0013-1. Curr Hypertens Rep. 2003. PMID: 12530938 Review.
-
Noradrenaline-induced changes in intracellular Ca(2+) and tension in mesenteric arteries from diabetic rats.Br J Pharmacol. 2001 Sep;134(1):179-87. doi: 10.1038/sj.bjp.0704221. Br J Pharmacol. 2001. PMID: 11522610 Free PMC article.
-
Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F(2alpha)-induced contraction of rabbit aortae.J Physiol. 2003 Feb 1;546(Pt 3):823-36. doi: 10.1113/jphysiol.2002.030775. J Physiol. 2003. PMID: 12563007 Free PMC article.
-
CPI-17-deficient smooth muscle of chicken.J Physiol. 2004 Jun 1;557(Pt 2):515-28. doi: 10.1113/jphysiol.2004.064543. Epub 2004 Apr 16. J Physiol. 2004. PMID: 15090608 Free PMC article.
References
-
- Akata T. Possible involvement of guanosine-5′-triphosphate in calcium-activation of contractile proteins in vascular smooth muscle. Journal of Vascular Research. 1996;33(suppl. 2):30. (abstract)
-
- Brozovich FV. PKC regulates agonist-induced force enhancement in single alpha-toxin-permeabilized vascular smooth muscle cells. American Journal of Physiology. 1995;268:C1202–1206. - PubMed
-
- Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochemical and Biophysical Research Communications. 1991;176:288–293. - PubMed
-
- Chen YH, Chen MX, Alessi DR, Campbell DG, Shanahan C, Cohen P, Cohen PTW. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1 M. FEBS Letters. 1994;356:51–55. - PubMed
-
- Coburn RF, Azim S, Fillers WS, Baron CB. Smooth muscle guanine nucleotides and receptor-effector coupling following inhibition of oxidative energy production. American Journal of Physiology. 1993;264:L1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

