Modification of C1- transport in skeletal muscle of Rana temporaria with the arginine-binding reagent phenylglyoxal
- PMID: 9706006
- PMCID: PMC2231055
- DOI: 10.1111/j.1469-7793.1998.591bk.x
Modification of C1- transport in skeletal muscle of Rana temporaria with the arginine-binding reagent phenylglyoxal
Abstract
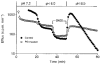
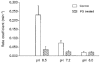
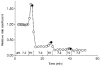
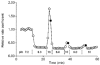
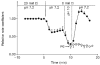
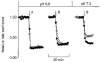
1. The effect of membrane modification by the arginine-binding reagent phenylglyoxal (PG) on Cl- permeability was studied in thin bundles of twitch fibres from frog muscle. The bundles were modified by a method that yields stable PG binding to outer arginyl residues in erythrocyte membranes. 2. PG almost eliminated the pH-dependent fraction of 36Cl- efflux under conditions of Cl- equilibrium in depolarized bundles: the fluxes at pH 7.2 and 8.5 were strongly inhibited approaching an apparent baseline value close to the normal flux at pH 6 which per se was not inhibited. 3. The uninhibited flux at pH 6 in modified bundles maintained the normally high sensitivity to 4,4'-dinitro-stilbene-2,2'-disulphonate (DNDS), and the reduction of fluxes at pH > 7 coincided with increased DNDS sensitivity, suggesting a selective blocking of the pH-dependent flux fraction that has a low DNDS sensitivity. 4. In normal Ringer solution the modified fibres showed normal resting membrane potentials (Vm) with normal sensitivity to [K+]o but sensitivity to changes of [Cl-]o was almost eliminated, suggesting a normal resting Na+:K+ conductance ratio (gNa/gK) and that the main influence of modification on the resting membrane conductance (gm) was a loss of Cl- conductance (gCl). 5. The modified fibres were not excitable, possibly due to arginine modification in the voltage sensor (S4) of the Na+ channels. 6. These results suggest that positively charged arginines are important for the activity of the pH-dependent Vm-stabilizing Cl- channels and that PG may isolate a pH-independent basal flux fraction which normally dominates the Cl- flux at low pH.
Figures



References
-
- Bjerrum PJ. Chemical modification of the anion-transport system with phenylglyoxal. Methods in Enzymology. 1989;173:466–494. - PubMed
-
- Bretag AH. Muscle chloride channels. Physiological Reviews. 1987;67:613–724. - PubMed
-
- Cheung ST, Fonda ML. Reaction of phenylglyoxal with arginine. The effect of buffers and pH. Biochemical and Biophysical Research Communications. 1979;90:940–947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

