Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C
- PMID: 9706014
- PMCID: PMC2231142
- DOI: 10.1111/j.1469-7793.1998.333bh.x
Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C
Abstract
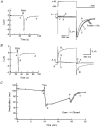
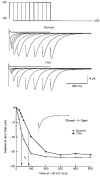
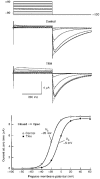
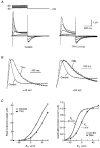
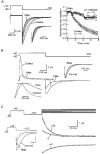
1. Modulation of the human ether-à-go-go-related gene (HERG) K+ channel was studied in two-electrode voltage-clamped Xenopus oocytes co-expressing the channel protein and the thyrotropin-releasing hormone (TRH) receptor. 2. Addition of TRH caused clear modifications of HERG channel gating kinetics. These variations consisted of an acceleration of deactivation, as shown by a faster decay of hyperpolarization-induced tail currents, and a slower time course of activation, measured using an envelope of tails protocol. The voltage dependence for activation was also shifted by nearly 20 mV in the depolarizing direction. Neither the inactivation nor the inactivation recovery rates were altered by TRH. 3. The alterations in activation gating parameters induced by TRH were demonstrated in a direct way by looking at the increased outward K+ currents elicited in extracellular solutions in which K+ was replaced by Cs+. 4. The effects of TRH were mimicked by direct pharmacological activation of protein kinase C (PKC) with beta-phorbol 12-myristate, 13-acetate (PMA). The TRH-induced effects were antagonized by GF109203X, a highly specific inhibitor of PKC that also abolished the PMA-dependent regulation of the channels. 5. It is concluded that a PKC-dependent pathway links G protein-coupled receptors that activate phospholipase C to modulation of HERG channel gating. This provides a mechanism for the physiological regulation of cardiac function by phospholipase C-activating receptors, and for modulation of adenohypophysial neurosecretion in response to TRH.
Figures


References
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Barros F, del Camino D, Pardo LA, Palomero T, Giráldez T, de la Peña P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflügers Archiv. 1997;435:119–129. - PubMed
-
- Barros F, Delgado LM, Del Camino D, de la Peña P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflügers Archiv. 1992;422:31–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

