Effect of depletion of glycosaminoglycans and non-collagenous proteins on interstitial hydraulic permeability in rabbit synovium
- PMID: 9706037
- PMCID: PMC2231131
- DOI: 10.1111/j.1469-7793.1998.629bh.x
Effect of depletion of glycosaminoglycans and non-collagenous proteins on interstitial hydraulic permeability in rabbit synovium
Abstract
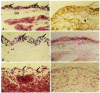
1. The hydraulic resistance of synovial interstitium helps to retain a lubricating fluid within the joint cavity. The contributions of sulphated glycosaminoglycans to resistance were assessed by selective depletion by chondroitinase ABC, keratanase and heparinases I, II and III in vivo. Also, since glycosaminoglycans do not account fully for the resistance, the contribution of non-collagenous, structural proteins in interstitium was assessed by treatment with chymopapain, a collagen-sparing protease. 2. Ringer solution containing enzyme was injected into the synovial cavity of the knee in anaesthetized rabbits. After >= 30 min the intra-articular pressure was raised and the relation between pressure (Pj) and trans-synovial outflow (Qs) determined. The slope dQs/dPj at low pressures, i.e. below yield pressure, represents the hydraulic conductance of the lining, i.e. 1/resistance. The contralateral joint received Ringer solution without enzyme as a control. Action of enzymes on the tissue was confirmed by histochemical and immunohistochemical studies. 3. Treatment with chondroitinase ABC (5 joints) increased the hydraulic conductance of the lining by 2.3 times (control, 1.34 +/- 0.22 microliter l min-1 cmH2O-1; post-enzyme, 3.11 +/- 0.45 microliter l min-1 cmH2O-1). This was significantly less than the effects of leech, Streptomyces and testicular hyaluronidases, which caused an average 4.7 times increase (P < 0.001, ANOVA). Analogous findings were made above yield pressure. 4. Treatment with keratanase (3 joints) or heparinases I, II and III (3 joints) caused no significant increase in trans-synovial flows or conductance, even though the concentration of heparan sulphate in synovium is higher than that of chondroitin sulphates or hyaluronan. 5. Treatment with chymopapain (7 joints) caused the greatest increases in trans-synovial flow, which exceeded control flow by an order of magnitude in one case. After 0.1 U chymopapain the average conductance was 6.6 times the control conductance below yield pressure. Immunohistochemical studies confirmed that chymopapain treatment removed the synovial proteoglycans. 6. It is concluded that, despite their similar resistivities in vitro, the different glycosaminoglycans do not contribute equally, weight for weight, to interstitial resistance in vivo. Hyaluronan is the dominant glycosaminoglycan governing synovial interstitial resistance. In addition, non-collagenous structural proteins contribute significantly to interstitial resistance.
Figures


 ) or without chondroitinase ABC pre-treatment (□) in pairs of knees from five rabbits (means ±
) or without chondroitinase ABC pre-treatment (□) in pairs of knees from five rabbits (means ±
 ) or without keratanase pre-treatment (□) in pairs of knees from three rabbits (means ±
) or without keratanase pre-treatment (□) in pairs of knees from three rabbits (means ±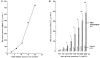
 ) and in control joints (□; Student's t test, *P≤ 0.05, **P < = 0.01).
) and in control joints (□; Student's t test, *P≤ 0.05, **P < = 0.01).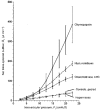
References
-
- Bert JL, Reed RK. Hyaluronan and the flow conductivity of rat dermis. In: Reed RK, Rubin K, editors. Connective Tissue Biology, Integration and Reductionism. London: Portland Press; 1998. pp. 41–48.
-
- Bradford DS, Oegema TR, Cooper KM, Wakano K, Chao EY. Chymopapain, chemonucleolysis and nucleus pulposus regeneration. A biochemical and biomechanical study. Spine. 1984;9:135–147. - PubMed
-
- Buchmann MD, Gluzband YA, Grodzinsky AJ, Hunzicker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. Journal of Cell Science. 1995;108:1497–1508. - PubMed
-
- Caterson B, Christner JE, Baker JR, Couchman D. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings. 1985;44:386–393. - PubMed
-
- Coleman PJ, Kavanagh E, Mason RM, Levick JR, Ashhurst DE. The proteoglycans and glycosaminoglycan chains of rabbit synovium. Histochemical Journal. 1998a in the Press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

