DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone
- PMID: 9707545
- PMCID: PMC21406
- DOI: 10.1073/pnas.95.17.9738
DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone
Abstract
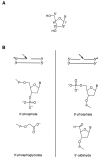
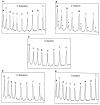
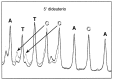
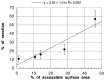
Despite extensive study, there is little experimental information available as to which of the deoxyribose hydrogen atoms of duplex DNA reacts most with the hydroxyl radical. To investigate this question, we prepared a set of double-stranded DNA molecules in which deuterium had been incorporated specifically at each position in the deoxyribose of one of the four nucleotides. We then measured deuterium kinetic isotope effects on the rate of cleavage of DNA by the hydroxyl radical. These experiments demonstrate that the hydroxyl radical reacts with the various hydrogen atoms of the deoxyribose in the order 5' H > 4' H > 3' H approximately 2' H approximately 1' H. This order of reactivity parallels the exposure to solvent of the deoxyribose hydrogens. Our work therefore reveals the structural basis of the reaction of the hydroxyl radical with DNA. These results also provide information on the mechanism of DNA damage caused by ionizing radiation as well as atomic-level detail for the interpretation of hydroxyl radical footprints of DNA-protein complexes and chemical probe experiments on the structure of RNA and DNA in solution.
Figures
References
-
- von Sonntag C. The Chemical Basis of Radiation Biology. London: Taylor & Francis; 1987.
-
- Tullius T D. Nature (London) 1988;332:663–664. - PubMed
-
- Lee S, Hahn S. Nature (London) 1995;376:609–612. - PubMed
-
- Price M A, Tullius T D. Methods Enzymol. 1992;212:194–219. - PubMed
-
- Latham J A, Cech T R. Science. 1989;245:276–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

