Mechanism of capsid maturation in a double-stranded DNA virus
- PMID: 9707570
- PMCID: PMC21431
- DOI: 10.1073/pnas.95.17.9885
Mechanism of capsid maturation in a double-stranded DNA virus
Abstract
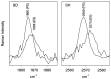
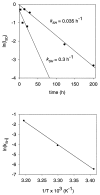
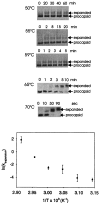
Folding mechanisms of proteins incorporated within supramolecular assemblies, including viruses, are little understood and may differ fundamentally from folding mechanisms of small globular proteins. We describe a novel Raman dynamic probe of hydrogen-isotope exchange to investigate directly these protein folding/assembly pathways. The method is applied to subunit folding in assembly intermediates of the double-stranded DNA bacteriophage P22. The icosahedral procapsid-to-capsid maturation (shell expansion) of P22 is shown to be accompanied by a large increase in exchange protection of peptide beta-strands. The molecular mechanism of shell expansion involves unfolding of metastable tertiary structure to form more stable quaternary contacts and is governed by a surprisingly high activation energy. The results demonstrate that coat subunit folding and capsid expansion are strongly coupled processes. Subunit structure in the procapsid represents a late intermediate along the folding/assembly pathway to the mature capsid. Coupling of folding and assembly is proposed as a general pathway for the construction of supramolecular complexes.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

