Mutation frequency declines during spermatogenesis in young mice but increases in old mice
- PMID: 9707592
- PMCID: PMC21453
- DOI: 10.1073/pnas.95.17.10015
Mutation frequency declines during spermatogenesis in young mice but increases in old mice
Abstract
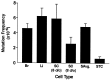
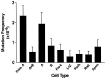
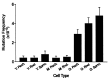
Five percent of live-born human offspring will have a genetic disorder. Of these, 20% are because of germ-line de novo mutations. Several genetic diseases, such as neurofibromatosis and Duchenne muscular dystrophy, are associated with a high percentage of de novo germ-line mutations. Until recently, a direct analysis of spontaneous mutation frequencies in mammalian germ cells has been prevented by technical limitations. We have measured spontaneous mutation frequencies in a lacI transgene by using enriched populations of specific spermatogenic cell types. Similar to previously published results, we observed a lower mutation frequency for seminiferous tubule cell preparations, which contain all stages of spermatogenesis, relative to somatic tissues. We made the unexpected observation of a decline in mutation frequency during spermatogenesis, such that the mutation frequencies of type B spermatogonia and all subsequent stages of spermatogenesis are lower than the frequency for primitive type A spermatogonia. In addition, spermatogenic cells from old mice have significantly increased mutation frequencies compared with spermatogenic cells from young or middle-aged mice. Finally, the mutation frequency was observed to increase during spermiogenesis in postreplicative cell types when spermatogenic cells were obtained from old mice.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

