CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma
- PMID: 9707601
- PMCID: PMC21462
- DOI: 10.1073/pnas.95.17.10067
CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma
Abstract
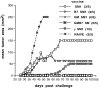
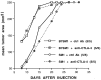
Generation of a T cell-mediated antitumor response depends on T cell receptor engagement by major histocompatibility complex/antigen as well as CD28 ligation by B7. CTLA-4 is a second B7 receptor expressed by T cells upon activation that, unlike CD28, appears to deliver an inhibitory signal to T cells. Recently, we and others demonstrated that administration of an anti-CTLA-4 antibody was sufficient to promote regression of several murine tumors. However, certain tumors, such as the SM1 mammary carcinoma, remain refractory to this type of immunotherapy. In the present study, we report that the combination of both CTLA-4 blockade and a vaccine consisting of granulocyte-macrophage colony-stimulating factor-expressing SM1 cells resulted in regression of parental SM1 tumors, despite the ineffectiveness of either treatment alone. This synergistic therapy resulted in long-lasting immunity to SM1 and depended on both CD4(+) and CD8(+) T cells. Interestingly, synergy was not observed between CTLA-4 and a B7-expressing SM1 vaccine. Given that granulocyte-macrophage colony-stimulating factor promotes differentiation and activation of dendritic cells as well as enhances cross-priming of T cells to tumor-derived antigens and that SM1 is major histocompatibility complex class II-negative, our findings suggest that CTLA-4 blockade acts at the level of a host-derived antigen-presenting cell. In addition, these results also support the idea that the most effective and synergistic vaccine strategy targets treatments that enhance T cell priming at the level of host-derived antigen-presenting cells.
Figures



References
-
- Allison J P. Curr Opin Immunol. 1994;6:414–419. - PubMed
-
- Chen L, Ashe S, Brady W A, Hellstrom I, Hellstrom K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. - PubMed
-
- Townsend S, Allison J P. Science. 1993;259:368–370. - PubMed
-
- Townsend S E, Su F W, Atherton J M, Allison J P. Cancer Res. 1994;54:6477–6483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

