The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis
- PMID: 9707607
- PMCID: PMC21468
- DOI: 10.1073/pnas.95.17.10100
The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis
Abstract
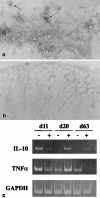
Glial growth factor 2 (GGF2) is a neuronal signal that promotes the proliferation and survival of the oligodendrocyte, the myelinating cell of the central nervous system (CNS). The present study examined whether recombinant human GGF2 (rhGGF2) could effect clinical recovery and repair to damaged myelin in chronic relapsing experimental autoimmune encephalomyelitis (EAE) in the mouse, a major animal model for the human demyelinating disease, multiple sclerosis. Mice with EAE were treated with rhGGF2 during both the acute and relapsing phases. Clinically, GGF2 treatment delayed signs, decreased severity, and resulted in statistically significant reductions in relapse rate. rhGGF2-treated groups displayed CNS lesions with more remyelination than in controls. This correlated with increased mRNA expression of myelin basic protein exon 2, a marker for remyelination, and with an increase in the CNS of the regulatory cytokine, interleukin 10, at both the RNA and protein levels. Thus, a beneficial effect of a neurotrophic growth factor has been demonstrated on the clinical, pathologic, and molecular manifestations of autoimmune demyelination, an effect that was associated with increased expression of a T helper 2 cytokine. rhGGF2 treatment may represent a novel approach to the treatment of multiple sclerosis.
Figures




References
-
- Goodkin D E. In: Multiple Sclerosis Clinical and Pathogenetic Basis. Raine C S, McFarland H F, Tourtellotte W W, editors. London: Chapman & Hall; 1997. pp. 307–324.
-
- Brosnan C F, Racke M K, Selmaj K. In: Multiple Sclerosis Clinical and Pathogenetic Basis. Raine C S, McFarland H F, Tourtellotte W W, editors. London: Chapman & Hall; 1997. pp. 325–340.
-
- Raine C S. In: Multiple Sclerosis Clinical and Pathogenetic Basis. Raine C S, McFarland H F, Tourtellotte W W, editors. London: Chapman & Hall; 1997. pp. 243–286.
-
- Prineas J W, McDonald W I. In: Greenfield’s Neuropathology. Graham D I, Lantos P L, editors. Oxford: Oxford Univ. Press; 1997. pp. 813–896.
-
- Mokhtarian F, McFarlin D E, Raine C S. Nature (London) 1984;309:356–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

