Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia
- PMID: 9707622
- PMCID: PMC21483
- DOI: 10.1073/pnas.95.17.10188
Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia
Abstract
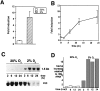
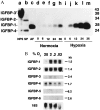
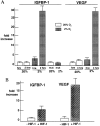
IGFBP-1 is elevated in fetuses with long-term, chronic hypoxia and intrauterine growth restriction. We investigated the hypothesis that hypoxia regulates IGFBP-1 in the human fetus in vivo and IGFBP-1 gene expression and protein in vitro. Umbilical artery IGFBP-1 levels (mean +/- SEM) from term babies with respiratory acidosis (acute hypoxia), normal babies, and those with mixed respiratory/metabolic acidosis (more profound and prolonged hypoxia) were measured using an immunoradiometric assay. IGFBP-1 levels were similar in normal (n = 12) and acutely hypoxic (n = 6) babies (189.1 +/- 71.8 vs. 175.8 +/- 45.9 ng /ml, respectively, P = 0.789). However, with more profound and prolonged hypoxia (n = 19), IGFBP-1 levels were markedly elevated (470.6 +/- 80.0 ng /ml, P = 0.044). To investigate IGFBP-1 regulation by hypoxia in vitro, HepG2 cells were incubated under hypoxia (pO2 = 2%) and normoxia (pO2 = 20%). IGFBP-1 protein and mRNA increased 8- and 12-fold, respectively, under hypoxic conditions. Hypoxia did not affect protein or mRNA levels of IGFBP-2 or -4. IGFBP-5 and -6 mRNAs, undetectable in control cells, were not induced by hypoxia, whereas minimally expressed IGFBP-3 mRNA increased twofold. Investigation into IGFBP-1 gene structure revealed three potential consensus sequences for the hypoxia response element (HRE) in the first intron. To investigate functionality, a 372-bp fragment of IGFBP-1 intron 1, containing putative HREs, was placed 5' to a heterologous hsp70 promoter in a plasmid using luciferase as a reporter gene. Under hypoxia, reporter gene activity increased up to 30-fold. Mutations in the middle HRE abolished reporter activity in response to hypoxia, suggesting that this HRE is functional in the IGFBP-1 hypoxia response. Cotransfection of HRE reporter genes with a constitutively expressing hypoxia-inducible factor 1 plasmid in HepG2 cells resulted in a fourfold induction of reporter activity, suggesting a role for hypoxia-inducible factor 1 in hypoxia induction of IGFBP-1 gene expression. These data support the hypothesis that hypoxia regulation of IGFBP-1 may be a mechanism operating in the human fetus to restrict insulin-like growth factor-mediated growth in utero under conditions of chronic hypoxia and limited substrate availability.
Figures

References
-
- Creasy R K, Resnik R. In: Maternal-Fetal Medicine: Principles and Practice. Creasy R K, Resnik R, editors. Philadelphia: Saunders; 1993. pp. 547–563.
-
- Bang P, Giudice L C, Rosenfeld R G. Front Endocrinol. 1994;6:197–212.
-
- Chard T. Growth Regul. 1994;4:91–100. - PubMed
-
- Baker J, Liu J P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. - PubMed
-
- Woods K A, Camacho-Hubner C, Savage M O, Clark A J L. N Engl J Med. 1996;335:1363–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

