A genetic defect resulting in mild low-renin hypertension
- PMID: 9707624
- PMCID: PMC21485
- DOI: 10.1073/pnas.95.17.10200
A genetic defect resulting in mild low-renin hypertension
Abstract
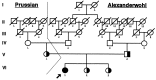
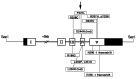
Severe low-renin hypertension has few known causes. Apparent mineralocorticoid excess (AME) is a genetic disorder that results in severe juvenile low-renin hypertension, hyporeninemia, hypoaldosteronemia, hypokalemic alkalosis, low birth weight, failure to thrive, poor growth, and in many cases nephrocalcinosis. In 1995, it was shown that mutations in the gene (HSD11B2) encoding the 11beta-hydroxysteroid dehydrogenase type 2 enzyme (11beta-HSD2) cause AME. Typical patients with AME have defective 11beta-HSD2 activity, as evidenced by an abnormal ratio of cortisol to cortisone metabolites and by an exceedingly diminished ability to convert [11-3H]cortisol to cortisone. Recently, we have studied an unusual patient with mild low-renin hypertension and a homozygous mutation in the HSD11B2 gene. The patient came from an inbred Mennonite family, and though the mutation identified her as a patient with AME, she did not demonstrate the typical features of AME. Biochemical analysis in this patient revealed a moderately elevated cortisol to cortisone metabolite ratio. The conversion of cortisol to cortisone was 58% compared with 0-6% in typical patients with AME whereas the normal conversion is 90-95%. Molecular analysis of the HSD11B2 gene of this patient showed a homozygous C-->T transition in the second nucleotide of codon 227, resulting in a substitution of proline with leucine (P227L). The parents and sibs were heterozygous for this mutation. In vitro expression studies showed an increase in the Km (300 nM) over normal (54 nM). Because approximately 40% of patients with essential hypertension demonstrate low renin, we suggest that such patients should undergo genetic analysis of the HSD11B2 gene.
Figures


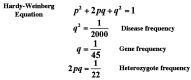
Similar articles
-
Prevalence of mild apparent mineralocorticoid excess in Mennonites.J Clin Endocrinol Metab. 1999 Dec;84(12):4735-8. doi: 10.1210/jcem.84.12.6340. J Clin Endocrinol Metab. 1999. PMID: 10599743
-
Human hypertension caused by mutations in the 11 beta-hydroxysteroid dehydrogenase gene: a molecular analysis of apparent mineralocorticoid excess.J Hypertens Suppl. 1996 Dec;14(5):S19-24. J Hypertens Suppl. 1996. PMID: 9120678
-
A mutation in the cofactor-binding domain of 11beta-hydroxysteroid dehydrogenase type 2 associated with mineralocorticoid hypertension.J Clin Endocrinol Metab. 2001 Mar;86(3):1247-52. doi: 10.1210/jcem.86.3.7334. J Clin Endocrinol Metab. 2001. PMID: 11238516
-
Apparent mineralocorticoid excess.Trends Endocrinol Metab. 2001 Apr;12(3):104-11. doi: 10.1016/s1043-2760(00)00356-8. Trends Endocrinol Metab. 2001. PMID: 11306334 Review.
-
The role of the 11beta-hydroxysteroid dehydrogenase type 2 in human hypertension.J Hypertens. 2000 Mar;18(3):241-8. doi: 10.1097/00004872-200018030-00001. J Hypertens. 2000. PMID: 10726708 Review.
Cited by
-
Apparent mineralocorticoid excess: comprehensive overview of molecular genetics.J Transl Med. 2022 Nov 3;20(1):500. doi: 10.1186/s12967-022-03698-9. J Transl Med. 2022. PMID: 36329487 Free PMC article. Review.
-
Clinical, genetic, and structural basis of apparent mineralocorticoid excess due to 11β-hydroxysteroid dehydrogenase type 2 deficiency.Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):E11248-E11256. doi: 10.1073/pnas.1716621115. Epub 2017 Dec 11. Proc Natl Acad Sci U S A. 2017. PMID: 29229831 Free PMC article.
-
A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22).J Med Genet. 2000 Nov;37(11):831-5. doi: 10.1136/jmg.37.11.831. J Med Genet. 2000. PMID: 11073536 Free PMC article.
-
Genetics of arterial hypertension and hypotension.Naunyn Schmiedebergs Arch Pharmacol. 2007 Feb;374(5-6):429-69. doi: 10.1007/s00210-007-0133-2. Epub 2007 Jan 30. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17262198 Review.
-
Mechanistic insights into the primary and secondary alterations of renal ion and water transport in the distal nephron.J Intern Med. 2023 Jan;293(1):4-22. doi: 10.1111/joim.13552. Epub 2022 Aug 21. J Intern Med. 2023. PMID: 35909256 Free PMC article. Review.
References
-
- New M I, Levine L S, Biglieri E G, Pareira J, Ulick S. J Clin Endocrinol Metab. 1977;44:924–933. - PubMed
-
- Dave-Sharma S, Wilson R C, Harbison M D, Newfield R, Razzaghy-Azar M, Krozowski Z, Funder J W, Shackleton C H L, Bradlow H L, Wei J, et al. J Clin Endocrinol Metab. 1998;83:2244–2254. - PubMed
-
- Wilson R C, Harbison M D, Krozowski Z S, Funder J W, Shackleton C H L, Hanauske-Able H M, Wei J Q, Hertecant J, Moran A, Neiberger R E, Balfe J W, et al. J Clin Endocrinol Metab. 1995;80:3145–3150. - PubMed
-
- Tannin G M, Agarwal A K, Monder C, New M I, White P C. J Biol Chem. 1991;266:16653–16658. - PubMed
-
- Whorwood C B, Mason J I, Rickets M L, Howie A J, Stewart P M. Mol Cell Endocrinol. 1995;110:R7–R11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

