The A kinase anchoring protein is required for mediating the effect of protein kinase A on ROMK1 channels
- PMID: 9707637
- PMCID: PMC21498
- DOI: 10.1073/pnas.95.17.10274
The A kinase anchoring protein is required for mediating the effect of protein kinase A on ROMK1 channels
Abstract
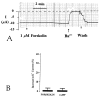
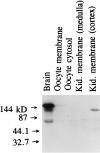
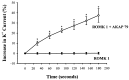
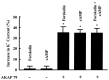
In the present study, we have used the two-electrode voltage-clamp and patch-clamp techniques to study the effects of forskolin and cAMP on the ROMK1 channels, which are believed to be the native K+ secretory channels in the kidney. Addition of 1 microM forskolin or 100 microM 8-bromo-cAMP, within 10 min, has no significant effect on the current of ROMK1 channels expressed in Xenopus oocytes. In contrast, application of 1 microM forskolin, within 3 min, significantly increased whole-cell K+ current by 35%, when ROMK1 channels were coexpressed with the A kinase anchoring protein AKAP79, which was cloned from neuronal tissue. Two lines of evidence indicate that the effect of forskolin is mediated by a cAMP-dependent pathway: (i) Addition of 100 microM 8-bromo-cAMP mimics the effect of forskolin and (ii) the effect of forskolin and cAMP is not additive. That AKAP is required for the effect of cAMP is further supported by experiments in which addition of ATP (100 microM) and cAMP (100 microM) restored the activity of run-down ROMK1 channels in inside-out patches in oocytes that coexpressed ROMK1 and AKAP79 but not in those that expressed ROMK1 alone. Moreover, when we used RII, the regulatory subunit of type II protein kinase A, in an overlay assay, we identified a RII-binding protein in membranes obtained from the kidney cortex but not in membranes from oocytes. This suggests that the insensitivity of ROMK1 channels to forskolin and cAMP is due to the absence of AKAPs. We conclude that AKAP may be a critical component that mediates the effect of protein kinase A on the ROMK channels in the kidney.
Figures



References
-
- Wang W H, Hebert S C, Giebisch G. Annu Rev Physiol. 1997;59:413–436. - PubMed
-
- Ho K, Nichols C G, Lederer W J, Lytton J, Vassilev P M, Kanazirska M V, Hebert S C. Nature (London) 1993;362:31–38. - PubMed
-
- Lee W S, Hebert S C. Am J Physiol. 1995;268:F1124–F1131. - PubMed
-
- Xu J Z, Hall A E, Peterson L N, Bienkowski M J, Eessalu T E, Hebert S C. Am J Physiol. 1997;273:F739–F748. - PubMed
-
- Chepilko S, Zhou H, Sackin H, Palmer L G. Am J Physiol. 1995;268:C389–C401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

