Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant
- PMID: 9707641
- PMCID: PMC21502
- DOI: 10.1073/pnas.95.17.10294
Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant
Abstract
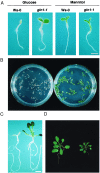
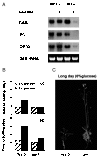
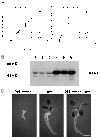
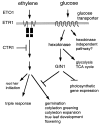
Glucose is an essential signaling molecule that controls plant development and gene expression through largely unknown mechanisms. To initiate the dissection of the glucose signal transduction pathway in plants by using a genetic approach, we have identified an Arabidopsis mutant, gin1 (glucose-insensitive), in which glucose repression of cotyledon greening and expansion, shoot development, floral transition, and gene expression is impaired. Genetic analysis indicates that GIN1 acts downstream of the sensor hexokinase in the glucose signaling pathway. Surprisingly, gin1 insensitivity to glucose repression of cotyledon and shoot development is phenocopied by ethylene precursor treatment of wild-type plants or by constitutive ethylene biosynthesis and constitutive ethylene signaling mutants. In contrast, the ethylene insensitive mutant etr1-1 exhibits glucose hypersensitivity. Epistasis analysis places GIN1 downstream of the ethylene receptor, ETR1, and defines a new branch of ethylene signaling pathway that is uncoupled from the triple response induced by ethylene. The isolation and characterization of gin1 reveal an unexpected convergence between the glucose and the ethylene signal transduction pathways. GIN1 may function to balance the control of plant development in response to metabolic and hormonal stimuli that act antagonistically.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

