Altering hemoglobin levels changes energy status in maize cells under hypoxia
- PMID: 9707645
- PMCID: PMC21506
- DOI: 10.1073/pnas.95.17.10317
Altering hemoglobin levels changes energy status in maize cells under hypoxia
Abstract
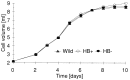
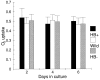
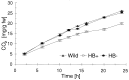
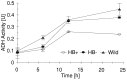
Nonsymbiotic hemoglobins are broadly present across the plant kingdom; however, the function of these proteins is unknown. Cultured maize cells have been transformed to constitutively express a barley hemoglobin gene in either the sense (HB+) or antisense (HB-) orientation. Hemoglobin protein in the transformed cell lines correspondingly was higher or lower than in wild-type cells under normal atmospheric conditions. Limiting oxygen availability, by placing the cells in a nitrogen atmosphere for 12 hr, had little effect on the energy status of cells constitutively expressing hemoglobin, but had a pronounced effect on both wild-type and HB- cells, where ATP levels declined by 27% and 61%, respectively. Total adenylates in these cells were approximately 35% lower than in HB+ cells. Energy charge was relatively unaffected by the treatment in HB+ and wild-type cells, but was reduced from 0.91 to 0.73 in HB- cells, suggesting that the latter were incapable of maintaining their energy status under the low oxygen regime. Treatment of the cells grown in an air atmosphere with antimycin A gave essentially the same results. It is suggested that nonsymbiotic hemoglobins act in plants to maintain the energy status of cells in low oxygen environments and that they accomplish this effect by promoting glycolytic flux through NADH oxidation, resulting in increased substrate-level phosphorylation. Hypoxic acclimation of plants is an example of this effect in nature. Nonsymbiotic hemoglobins are likely ancestors of an early form of hemoglobin that sequestered oxygen in low oxygen environments, providing a source of oxygen to oxidize NADH to provide ATP for cell growth and development.
Figures



References
-
- Wittenberg J B, Wittenberg B A. Annu Rev Biophys Biophys Chem. 1990;19:217–241. - PubMed
-
- Appleby C A. Sci Progress. 1992;76:365–398.
-
- Appleby C A. In: Nitrogen Fixation and CO2 Metabolism. Ludden P W, Burris J E, editors. New York: Elsevier Science; 1985. pp. 41–51.
-
- Duff S M G, Wittenberg J B, Hill R D. J Biol Chem. 1997;272:16746–16752. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

