A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists
- PMID: 9708319
- PMCID: PMC3459057
- DOI: 10.1080/07328319808004215
A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists
Abstract
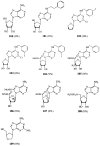
Various adenosine analogues were tested at the adenosine A2B receptor. Agonist potencies were determined by measuring the cyclic AMP production in Chinese Hamster Ovary cells expressing human A2B receptors. 5'-N-Substituted carboxamidoadenosines were most potent. 5'-N-Ethylcarboxamidoadenosine (NECA) was most active with an EC50 value of 3.1 microM. Other ribose modified derivatives displayed low to negligible activity. Potency was reduced by substitution on the exocyclic amino function (N6) of the purine ring system. The most active N6-substituted derivative N6-methyl-NECA was 5 fold less potent than NECA. C8- and most C2-substituted analogues were virtually inactive. 1-Deaza-analogues had a reduced potency, 3- and 7-deazaanalogues were not active.
Figures


Similar articles
-
A2B adenosine receptor agonists: synthesis and biological evaluation of 2-phenylhydroxypropynyl adenosine and NECA derivatives.Nucleosides Nucleotides Nucleic Acids. 2004;23(1-2):471-81. doi: 10.1081/ncn-120028340. Nucleosides Nucleotides Nucleic Acids. 2004. PMID: 15043167
-
Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells.Naunyn Schmiedebergs Arch Pharmacol. 1998 Jan;357(1):1-9. doi: 10.1007/pl00005131. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9459566
-
Characterization of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B4) cells.Br J Pharmacol. 1996 Nov;119(6):1286-90. doi: 10.1111/j.1476-5381.1996.tb16035.x. Br J Pharmacol. 1996. PMID: 8937736 Free PMC article.
-
Pharmacological characterization of adenosine A2B receptors: studies in human mast cells co-expressing A2A and A2B adenosine receptor subtypes.Biochem Pharmacol. 1998 Mar 1;55(5):627-33. doi: 10.1016/s0006-2952(97)00512-1. Biochem Pharmacol. 1998. PMID: 9515573
-
An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells.Br J Pharmacol. 1997 Oct;122(3):546-50. doi: 10.1038/sj.bjp.0701401. Br J Pharmacol. 1997. PMID: 9351513 Free PMC article.
Cited by
-
Use of the triazolotriazine [3H]ZM 241385 as a radioligand at recombinant human A2B adenosine receptors.Drug Des Discov. 1999 Nov;16(3):217-26. Drug Des Discov. 1999. PMID: 10624567 Free PMC article.
-
AMP579 is revealed to be a potent A2b-adenosine receptor agonist in human 293 cells and rabbit hearts.Basic Res Cardiol. 2010 Jan;105(1):129-37. doi: 10.1007/s00395-009-0056-9. Basic Res Cardiol. 2010. PMID: 19730798 Free PMC article.
-
1,3-Dialkylxanthine Derivatives Having High Potency as Antagonists at Human A2B Adenosine Receptors.Drug Dev Res. 1999 May;47(1):45-53. doi: 10.1002/(sici)1098-2299(199905)47:1<45::aid-ddr6>3.0.co;2-u. Drug Dev Res. 1999. PMID: 38239816 Free PMC article.
-
Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors.J Med Chem. 2000 Mar 23;43(6):1165-72. doi: 10.1021/jm990421v. J Med Chem. 2000. PMID: 10737749 Free PMC article.
-
The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion.Am J Physiol Gastrointest Liver Physiol. 2012 Feb 1;302(3):G397-405. doi: 10.1152/ajpgi.00087.2011. Epub 2011 Oct 28. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22038827 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
