A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists
- PMID: 9708319
- PMCID: PMC3459057
- DOI: 10.1080/07328319808004215
A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists
Abstract
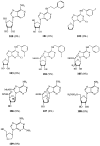
Various adenosine analogues were tested at the adenosine A2B receptor. Agonist potencies were determined by measuring the cyclic AMP production in Chinese Hamster Ovary cells expressing human A2B receptors. 5'-N-Substituted carboxamidoadenosines were most potent. 5'-N-Ethylcarboxamidoadenosine (NECA) was most active with an EC50 value of 3.1 microM. Other ribose modified derivatives displayed low to negligible activity. Potency was reduced by substitution on the exocyclic amino function (N6) of the purine ring system. The most active N6-substituted derivative N6-methyl-NECA was 5 fold less potent than NECA. C8- and most C2-substituted analogues were virtually inactive. 1-Deaza-analogues had a reduced potency, 3- and 7-deazaanalogues were not active.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
