Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance
- PMID: 9708802
- PMCID: PMC1852975
- DOI: 10.1016/S0002-9440(10)65585-X
Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance
Abstract
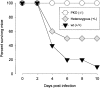
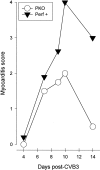
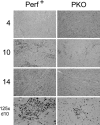
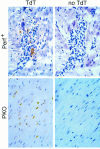
Viral myocarditis is remarkably common, being detected in approximately 1% of unselected asymptomatic individuals. Many cases are attributable to enteroviral infection, and in particular to coxsackievirus B3. The underlying pathogenesis is controversial, but most studies admit the important immunopathological role of infiltrating CD8+ (cytotoxic) T lymphocytes (CTLs). We have previously shown that CTLs play conflicting roles in coxsackievirus B (CVB) myocarditis; they assist in controlling virus replication, but also are instrumental in causing the extensive inflammatory disease, which often results in severe myocardial scarring. A role for perforin, the major CTL cytolytic protein, in CVB myocarditis has been suggested, but never proven. In the present study we use perforin knockout (PKO) mice to show that perforin plays a major role in CVB infection; in broad terms, perforin is important in immunopathology, but not in CVB clearance. For example, PKO mice are better able to withstand a normally lethal dose of CVB (100% survival of PKO mice compared with 90% death in +/+ littermates). In addition, PKO mice given a nonlethal dose of CVB develop only a mild myocarditis, whereas their perforin+ littermates have extensive myocardial lesions. The myocarditis in PKO mice resolves more quickly, and these mice show minimal histological sequelae; in contrast, late in disease the perforin+ mice develop severe myocardial fibrosis. PKO mice, despite lacking this major CTL effector function, can control the infection and eradicate the virus; growth kinetics and peak CVB titers are indistinguishable in PKO and perforin+ mice. Therefore, the immunopathological and antiviral effects of CTLs can be uncoupled by ablation of perforin; this offers a promising target for therapy of myocarditis. Furthermore, we evaluate the possible roles of apoptosis, and of chemokine expression, in CVB infection. In perforin+ mice, apoptotic cells are detected within the inflammatory infiltrate, whereas in their PKO counterparts, apoptotic myocyte nuclei are seen. Chemokine expression in both PKO and perforin+ mice precedes and parallels the course of myocarditis. Several chemokines are detectable earlier in PKO mice than in perforin+ mice, but PKO mice show reduced peak levels, and chemokine expression decays sooner. In particular, MIP-1alpha expression is barely detectable at any time point in PKO mice, but it is readily identified in perforin+ animals, peaking just before the time of maximal myocarditis; this is particularly interesting, given that MIP-1alpha knockout mice are resistant to CVB myocarditis, but remain able to control viral infection. Thus, the chemokine pathway offers a second route of intervention to diminish myocarditis and its sequelae, while permitting the host to eradicate the virus.
Figures




References
-
- Gravanis MB, Sternby NH: Incidence of myocarditis: a 10-year autopsy study from Malmo, Sweden. Arch Pathol Lab Med 1991, 115:390-392 - PubMed
-
- Sole MJ, Liu P: Viral myocarditis: a paradigm for understanding the pathogenesis and treatment of dilated cardiomyopathy. J Am Coll Cardiol 1993, 22:99A-105A - PubMed
-
- Goodwin JF: Cardiomyopathies and specific heart muscle diseases: definitions, terminology, classifications and new and old approaches. Postgrad Med J 1992, 68(suppl 1):S3-S6 - PubMed
-
- Heck CF, Shumway SJ, Kaye MP: The Registry of the International Society for Heart Transplantation: sixth official report, 1989. J Heart Transplant 1989, 8:271-276 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

