Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors
- PMID: 9708803
- PMCID: PMC1852969
- DOI: 10.1016/S0002-9440(10)65586-1
Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors
Abstract
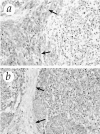
In this study, we investigated the expression patterns of 15 matrix metalloproteinases (MMPs) and three tissue inhibitors of metalloproteinase in gliomas, medulloblastomas, and normal brain tissue. By Northern blot analysis we found increased levels of mRNAs encoding for gelatinase A, gelatinase B, two membrane-type MMPs (mt1- and mt2-MMP), and tissue inhibitors of metalloproteinase-1 in glioblastomas and medulloblastomas. We observed a significant increase of mt1-MMP, gelatinase A, gelatinase B, and tissue inhibitors of metalloproteinase-1 in glioblastomas as compared with low-grade astrocytomas, anaplastic astrocytomas, and normal brain. In medulloblastomas, the expression of mt1-MMP, mt2-MMP, and gelatinase A were also increased, but to a lesser extent than that observed in glioblastomas. These data were confirmed at the protein level by immunostaining analysis. Moreover, substrate gel electrophoresis showed that the activated forms of gelatinases A and B were present in glioblastomas and medulloblastomas. These results suggest that increased expression of mt1-MMP/gelatinase A is closely related to the malignant progression observed in gliomas. Furthermore, the present study demonstrates, to our knowledge for the first time, that medulloblastomas express high levels of MMP.
Figures





References
-
- Giles GG, Gonzales MF: Epidemiology of brain tumors and factors in prognosis. Kaye AH Laws ER eds. Brain Tumors, ch 4. 1995, :pp 47-67 Churchill Livingstone, New York
-
- Gold EB: Epidemiology of brain tumors. Lilenfeld AM eds. Reviews in Cancer Epidemiology, 1980, vol 1.:pp 245-292 Elsevier, North Holland, the Netherlands
-
- Cerame MA, Guthikonda M, Kohli CM: Extraneural metastases in gliosarcomas: a case report and review of literature. Neurosurgery 1985, 17:413-418 - PubMed
-
- Rutka JT, Dougherty DV, Giblin JR, Edwards MS, McCulloch JR, Rosenblum ML: Growth of a medulloblastoma on normal leptomeningeal cells in culture: interaction of tumor cells and normal cells. Neurosurgery 1987, 21:872-878 - PubMed
-
- Berger MS, Magrassi L, Geyer R: Medulloblastomas and primitive neuroectodermal tumors. Kaye AH Laws ER eds. Brain Tumors, ch 30. 1995, :pp 562-574 Churchill Livingstone, New York
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

