Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus
- PMID: 9708805
- PMCID: PMC1852973
- DOI: 10.1016/S0002-9440(10)65588-5
Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus
Abstract
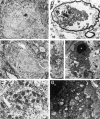
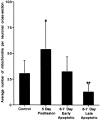
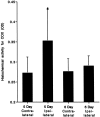
The mechanisms for neuronal apoptosis after axotomy and target deprivation in the adult central nervous system are poorly understood. We used a unilateral occipital cortex ablation model in the adult rat to test the hypothesis that apoptotic retrograde neurodegeneration in the dorsal lateral geniculate nucleus occurs in association with oxidative stress and mitochondrial abnormalities. Immunodetection of 8-hydroxy-2'-deoxyguanosine, a marker for oxidative injury to DNA, demonstrated that these apoptotic neurons undergo oxidative stress. Dual immunolabeling for the retrograde tracer Fluorogold to identify projection neurons and for 8-hydroxy-2'-deoxyguanosine demonstrated that apoptotic, oxidatively damaged neurons are geniculocortical projection neurons. By electron microscopy, degeneration of dorsal lateral geniculate nucleus neurons evolved in association with a transient increase in mitochondria within the perikaryon of dying neurons during the transition between chromatolysis and early apoptosis. The morphological integrity of mitochondria was preserved until late in the progression of apoptosis. The dorsal lateral geniculate nucleus ipsilateral to the cortical lesion had a transient increase in cytochrome c oxidase activity, and geniculocortical neurons at the transitional, early apoptotic stage accumulated cytochrome c oxidase activity. We conclude that axotomy-induced, retrograde neuronal apoptosis in the adult central nervous system occurs in association with the accumulation of functionally active mitochondria within the perikaryon and oxidative damage to nuclear DNA.
Figures


References
-
- Ratan RR, Murphy TH, Baraban JM: Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem 1994, 62:376-379 - PubMed
-
- Newmeyer DD, Farschon DM, Reed JC: Cell-free apoptosis in Xenopus egg extracts: inhibition by bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 1994, 79:353-364 - PubMed
-
- Skulachev VP: Why are mitochondria involved in apoptosis? FEBS Lett 1996, 397:7-10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

