Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia
- PMID: 9708812
- PMCID: PMC1852970
- DOI: 10.1016/S0002-9440(10)65595-2
Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia
Abstract
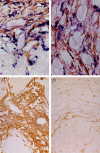
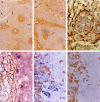
Extrahepatic biliary atresia is a severe neonatal liver disease resulting from a sclerosing cholangiopathy of unknown etiology. Although biliary obstruction may be surgically corrected by a "Kasai" hepatoportoenterostomy, most patients still develop progressive hepatic fibrosis, although the source of increased collagen deposition is unclear. This study examined the role of hepatic stellate cells (HSCs) and assessed the source of transforming growth factor-beta (TGF-beta) production in hepatic fibrogenesis in patients with biliary atresia. Liver biopsies from 18 biliary atresia patients (including 5 pre- and post-Kasai) were subjected to immunohistochemistry for alpha-smooth muscle actin and in situ hybridization for either procollagen alpha1 (I) mRNA or TGF-beta1 mRNA. Sections were also subjected to immunohistochemistry for active TGF-beta1 protein. The role of Kupffer cells in TGF-beta1 production was assessed by immunohistochemistry for CD68. Procollagen alpha1 (I) mRNA was colocalized to alpha-smooth muscle actin-positive HSCs within the region of increased collagen protein deposition in fibrotic septa and surrounding hyperplastic bile ducts. The number of activated HSCs was decreased in only one post-Kasai biopsy. TGF-beta1 mRNA expression was demonstrated in bile duct epithelial cells and activated HSCs and in hepatocytes in close proximity to fibrotic septa. Active TGF-beta1 protein was demonstrated in bile duct epithelial cells and activated HSCs. This study provides evidence that activated HSCs are responsible for increased collagen production in patients with biliary atresia and therefore play a definitive role in the fibrogenic process. We have also shown that bile duct epithelial cells, HSCs, and hepatocytes are all involved in the production of the profibrogenic cytokine, TGF-beta1.
Figures

References
-
- Balistreri WF: Neonatal cholestasis: medical progress. J Pediatr 1985, 106:171-184 - PubMed
-
- Kasai M, Yakovac WC, Koop CE: Liver in congenital biliary atresia and neonatal hepatitis: a histopathological study. Arch Pathol 1962, 74:152-162 - PubMed
-
- Kasai M, Suzuki S: A new operation for “non-correctable” biliary atresia: hepatic portoenterostomy. Shujutsu 1959, 13:733-739
-
- Kasai M: Treatment of biliary atresia with special reference to hepatic portoenterostomy and its modifications. Prog Pediatr Surg 1974, 6:5-52 - PubMed
-
- Otte JB, de Ville de Goyet J, Reding R, Hausleithner V, Sokal E, Chardot C, Debande B: Sequential treatment of biliary atresia with Kasai portoenterostomy, and liver transplantation: a review. Hepatology 1994, 20(Suppl):41S-48S - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

