Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism
- PMID: 9708819
- PMCID: PMC1852979
- DOI: 10.1016/S0002-9440(10)65602-7
Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism
Abstract
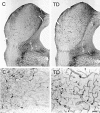
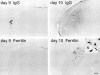
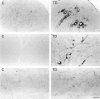
Abnormal oxidative processes including a reduction in thiamine-dependent enzymes accompany many neurodegenerative diseases. Thiamine deficiency (TD) models the cellular and molecular mechanisms by which chronic oxidative aberrations associated with thiamine-dependent enzyme deficits cause selective neurodegeneration. The mechanisms underlying selective cell death in TD are unknown. In rodent TD, the earliest region-specific pathological change is breakdown of the blood-brain barrier (BBB). The current studies tested whether nitric oxide and microglia are important in the initial events that couple BBB breakdown to selective neuronal loss. Enhanced expression of endothelial nitric oxide synthase and nicotinamide adenine dinucleotide phosphate diaphorase reactivity in microvessels, as well as the presence of numerous inducible nitric oxide synthase-immunoreactive microglia, accompanied the increases in BBB permeability. Nitric oxide synthase induction appears critical to TD pathology, because immunoreactivity for nitrotyrosine, a specific nitration product of peroxynitrite, also increased in axons of susceptible regions. In addition, TD elevated iron and the antioxidant protein ferritin in microvessels and in activated microglia, suggesting that these cells are responding to an oxidative challenge. All of these changes occurred in selectively vulnerable regions, preceding neuronal death. These findings are consistent with the hypothesis that the free radical-mediated BBB alterations permit entry of iron and extraneuronal proteins that set in motion a cascade of inflammatory responses culminating in selective neuronal loss. Thus, the TD model should help elucidate the relationship between oxidative deficits, BBB abnormalities, the inflammatory response, ferritin and iron elevation, and selective neurodegeneration.
Figures




References
-
- Blass JP, Gibson GE: The role of oxidative abnormalities in the pathophysiology of Alzheimer’s disease. Rev Neurol (Paris) 1991, 147:513. - PubMed
-
- Nixon RA, Cataldo AM: Free radicals, proteolysis and degeneration of neurons in Alzheimer’s disease: how essential is the β-amyloid link? Neurobiol Aging 1994, 15:463-469 - PubMed
-
- Smith MA, Perry G: Free radical damage, iron, and Alzheimer’s disease. J Neurol Sci 1995, 134:92-94 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

