Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter
- PMID: 9710594
- PMCID: PMC109095
- DOI: 10.1128/MCB.18.9.5099
Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter
Abstract
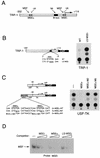
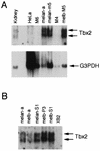
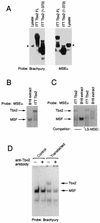
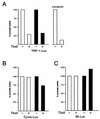
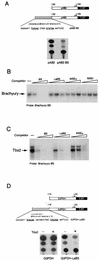
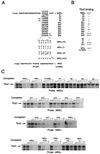
Previous work has demonstrated that two key melanocyte-specific elements termed the MSEu and MSEi play critical roles in the expression of the melanocyte-specific tyrosinase-related protein 1 (TRP-1) promoter. Both the MSEu and MSEi, located at position -237 and at the initiator, respectively, bind a melanocyte-specific factor termed MSF but are also recognized by a previously uncharacterized repressor, since mutations affecting either of these elements result in strong up-regulation of TRP-1 promoter activity in melanoma cells. Here we demonstrate that repression mediated by the MSEu and MSEi also operates in melanocytes. We also report that both the MSEu and MSEi are recognized by the brachyury-related transcription factor Tbx2, a member of the recently described T-box family, and that Tbx2 is expressed in melanocyte and melanoblast cell lines but not in melanoblast precursor cells. Although Tbx2 and MSF each recognize the TRP-1 MSEu and MSEi motifs, it is binding by Tbx-2, not binding by MSF, that correlates with repression. Several lines of evidence tend to point to the brachyury-related transcription factor Tbx2 as being the repressor of TRP-1 expression: both the MSEu and MSEi bind Tbx2, and mutations in either element that result in derepression of the TRP-1 promoter diminish binding by Tbx2; the TRP-1 promoter, but not the tyrosinase, microphthalmia, or glyceraldehyde-3-phosphate dehydrogenase (G3PDH) promoter, is repressed by Tbx2 in cotransfection assays; a high-affinity consensus brachyury/Tbx2-binding site is able to constitutively repress expression of the heterologous IE110 promoter; and a low-affinity brachyury/Tbx2 binding site is able to mediate Tbx2-dependent repression of the G3PDH promoter. Although we cannot rule out the presence of an additional, as yet unidentified factor playing a role in the negative regulation of TRP-1 in vivo, the evidence presented here suggests that Tbx2 most likely is the previously unidentified repressor of TRP-1 expression and as such is likely to represent the first example of transcriptional repression by a T-box family member.
Figures
References
-
- Baldwin C T, Lipsky N R, Hoth C F, Cohen T, Mamuya W, Milunsky A. Mutations in PAX3 associated with Waardenburg syndrome type I. Hum Mutat. 1994;3:205–211. - PubMed
-
- Bassi M T, Incerti B, Easty D J, Sviderskaya E V, Ballabio A. Cloning of the murine homolog of the ocular albinism type 1 (OA1) gene: sequence, genomic structure, and expression analysis in pigment cells. Genome Res. 1996;6:880–885. - PubMed
-
- Baynash A G, Hosoda K, Giaid A, Richardson J A, Emoto N, Hammer R E, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. - PubMed
-
- Bennett, D. Personal communication.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
