Genetic, physical, and functional interactions between the triphosphatase and guanylyltransferase components of the yeast mRNA capping apparatus
- PMID: 9710603
- PMCID: PMC109104
- DOI: 10.1128/MCB.18.9.5189
Genetic, physical, and functional interactions between the triphosphatase and guanylyltransferase components of the yeast mRNA capping apparatus
Abstract
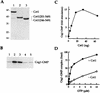
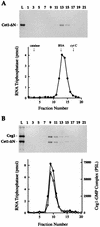
We have characterized an essential Saccharomyces cerevisiae gene, CES5, that when present in high copy, suppresses the temperature-sensitive growth defect caused by the ceg1-25 mutation of the yeast mRNA guanylyltransferase (capping enzyme). CES5 is identical to CET1, which encodes the RNA triphosphatase component of the yeast capping apparatus. Purified recombinant Cet1 catalyzes hydrolysis of the gamma phosphate of triphosphate-terminated RNA at a rate of 1 s-1. Cet1 is a monomer in solution; it binds with recombinant Ceg1 in vitro to form a Cet1-Ceg1 heterodimer. The interaction of Cet1 with Ceg1 elicits >10-fold stimulation of the guanylyltransferase activity of Ceg1. This stimulation is the result of increased affinity for the GTP substrate. A truncated protein, Cet1(201-549), has RNA triphosphatase activity, heterodimerizes with and stimulates Ceg1 in vitro, and suffices when expressed in single copy for cell growth in vivo. The more extensively truncated derivative Cet1(246-549) also has RNA triphosphatase activity but fails to stimulate Ceg1 in vitro and is lethal when expressed in single copy in vivo. These data suggest that the Cet1-Ceg1 interaction is essential but do not resolve whether the triphosphatase activity is also necessary. The mammalian capping enzyme Mce1 (a bifunctional triphosphatase-guanylyltransferase) substitutes for Cet1 in vivo. A mutation of the triphosphatase active-site cysteine of Mce1 is lethal. Hence, an RNA triphosphatase activity is essential for eukaryotic cell growth. This work highlights the potential for regulating mRNA cap formation through protein-protein interactions.
Figures


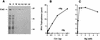
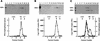
References
-
- Cong P, Shuman S. Covalent catalysis in nucleotidyl transfer: a KTDG motif essential for enzyme-GMP complex formation by mRNA capping enzyme is conserved at the active sites of RNA and DNA ligases. J Biol Chem. 1993;268:7256–7260. - PubMed
-
- Hakansson K, Doherty A J, Shuman S, Wigley D B. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell. 1997;89:545–553. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
