Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation
- PMID: 9710605
- PMCID: PMC109106
- DOI: 10.1128/MCB.18.9.5208
Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation
Abstract
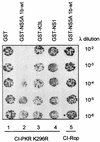
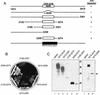
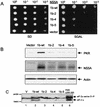
The PKR protein kinase is a critical component of the cellular antiviral and antiproliferative responses induced by interferons. Recent evidence indicates that the nonstructural 5A (NS5A) protein of hepatitis C virus (HCV) can repress PKR function in vivo, possibly allowing HCV to escape the antiviral effects of interferon. NS5A presents a unique tool by which to study the molecular mechanisms of PKR regulation in that mutations within a region of NS5A, termed the interferon sensitivity-determining region (ISDR), are associated with sensitivity of HCV to the antiviral effects of interferon. In this study, we investigated the mechanisms of NS5A-mediated PKR regulation and the effect of ISDR mutations on this regulatory process. We observed that the NS5A ISDR, though necessary, was not sufficient for PKR interactions; we found that an additional 26 amino acids (aa) carboxyl to the ISDR were required for NS5A-PKR complex formation. Conversely, we localized NS5A binding to within PKR aa 244 to 296, recently recognized as a PKR dimerization domain. Consistent with this observation, we found that NS5A from interferon-resistant HCV genotype 1b disrupted kinase dimerization in vivo. NS5A-mediated disruption of PKR dimerization resulted in repression of PKR function and inhibition of PKR-mediated eIF-2alpha phosphorylation. Introduction of multiple ISDR mutations abrogated the ability of NS5A to bind to PKR in mammalian cells and to inhibit PKR in a yeast functional assay. These results indicate that mutations within the PKR-binding region of NS5A, including those within the ISDR, can disrupt the NS5A-PKR interaction, possibly rendering HCV sensitive to the antiviral effects of interferon. We propose a model of PKR regulation by NS5A which may have implications for therapeutic strategies against HCV.
Figures


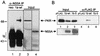
References
-
- Alter M. Epidemiology of hepatitis C in the west. Semin Liver Dis. 1995;15:5–14. - PubMed
-
- Barber G N, Jagus R, Meurs E F, Hovanessian A G, Katze M G. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
