14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity
- PMID: 9710607
- PMCID: PMC109108
- DOI: 10.1128/MCB.18.9.5229
14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity
Abstract
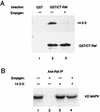
By binding to serine-phosphorylated proteins, 14-3-3 proteins function as effectors of serine phosphorylation. The exact mechanism of their action is, however, still largely unknown. Here we demonstrate a requirement for 14-3-3 for Raf-1 kinase activity and phosphorylation. Expression of dominant negative forms of 14-3-3 resulted in the loss of a critical Raf-1 phosphorylation, while overexpression of 14-3-3 resulted in enhanced phosphorylation of this site. 14-3-3 levels, therefore, regulate the stoichiometry of Raf-1 phosphorylation and its potential activity in the cell. Phosphorylation of Raf-1, however, was insufficient by itself for kinase activity. Removal of 14-3-3 from phosphorylated Raf abrogated kinase activity, whereas addition of 14-3-3 restored it. This supports a paradigm in which the effects of phosphorylation on serine as well as tyrosine residues are mediated by inducible protein-protein interactions.
Figures


References
-
- Aitken A. 14-3-3 proteins on the MAP. Trends Biochem Sci. 1995;20:95–97. - PubMed
-
- Autieri M V, Haines D S, Romanic A M, Ohlstein E H. Expression of 14-3-3 gamma in injured arteries and growth factor- and cytokine-stimulated human vascular smooth muscle cells. Cell Growth Differ. 1996;7:1453–1460. - PubMed
-
- Avruch J, Zhang X F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
-
- Boston P, Jackson P, Kynoch P, Thompson R. Purification, properties and immunohistochemical localisation of human brain 14-3-3 protein. J Neurochem. 1982;38:1466–1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
