Fos family members induce cell cycle entry by activating cyclin D1
- PMID: 9710644
- PMCID: PMC109145
- DOI: 10.1128/MCB.18.9.5609
Fos family members induce cell cycle entry by activating cyclin D1
Abstract
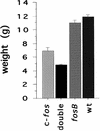
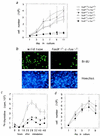
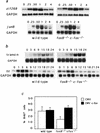
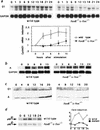
Expression of the fos family of transcription factors is stimulated by growth factors that induce quiescent cells to reenter the cell cycle, but the cellular targets of the Fos family that regulate cell cycle reentry have not been identified. To address this issue, mice that lack two members of the fos family, c-fos and fosB, were derived. The fosB-/- c-fos-/- mice are similar in phenotype to c-fos-/- mice but are 30% smaller. This decrease in size is consistent with an abnormality in cell proliferation. Fibroblasts derived from fosB-/- c-fos-/- mice were found to have a defect in proliferation that results at least in part from a failure to induce cyclin D1 following serum-stimulated cell cycle reentry. Although definitive evidence that c-Fos and FosB directly induce cyclin D1 transcription will require further analysis, these findings raise the possibility that c-Fos and FosB are either direct or indirect transcriptional regulators of the cyclin D1 gene and may function as a critical link between serum stimulation and cell cycle progression.
Figures

References
-
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Brown J R, Ye H, Bronson R T, Dikkes P, Greenberg M E. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. - PubMed
-
- Bruesselbach S, Moehle-Steinlein U, Wang Z-Q, Schreiber M, Lucibello F C, Mueller R, Wagner E F. Cell proliferation and cell cycle progression are not impaired in fibroblasts and ES cells lacking c-Fos. Oncogene. 1995;10:79–86. - PubMed
-
- Busch S J, Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990;6:36–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
