Regulation of the antibody repertoire through control of HCDR3 diversity
- PMID: 9711776
- PMCID: PMC4282159
- DOI: 10.1016/s0264-410x(98)00096-6
Regulation of the antibody repertoire through control of HCDR3 diversity
Abstract
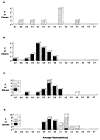
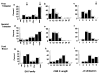
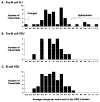
In man, as in mouse, diversification of the antibody repertoire appears to follow a strict developmental program whereby antigen specificities are serially acquired during ontogeny. When compared to the adult repertoire, the fetal antibody repertoire is highly enriched for polyreactive specificities of low affinity. Although the mechanisms governing the development of this fetal repertoire differ between human and mouse, the composition and structure of the fetal antibodies produced by both species are quite homologous. Specifically, both species use similar V gene segments and restrict the sequence and structure of the third complementarity determining region (HCDR3) of the antibody heavy chain. The precise role that this restriction of the HCDR3 might play in the development of immunocompetence in the human remains to be elucidated.
Figures
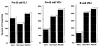
References
-
- Paton JC, Toogood IR, Cockington RA, Hansman DJ. Antibody response to pneumococcal vaccine in children aged 5 to 15 years. American Journal of Diseases of Childhood. 1986;140:135–138. - PubMed
-
- Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. Journal of Infectious Diseases. 1992;165:S49–S52. - PubMed
-
- Silverstein AM. Ontogeny of the immune response: a perspective. In: Cooper MD, editor. Development of Host Defense. 1. Raven Press; New York: 1977. pp. 1–10.
-
- Schroeder HW, Jr, Perlmutter RM. Development of the human antibody repertoire. In: Gupta S, Griscelli C, editors. New Concepts in Immunodeficiency Diseases. Chichester; 1993. pp. 1–20.
-
- Schroeder HW, Jr, Mortari F, Shiokawa S, Kirkham PM, Elgavish RA, Bertrand FEI. Developmental regulation of the human antibody repertoire. Annals of the New York Academy Sciences. 1995;764:242–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

