Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro
- PMID: 9712636
- PMCID: PMC6792984
- DOI: 10.1523/JNEUROSCI.18-17-06641.1998
Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro
Abstract
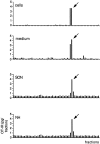
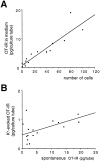
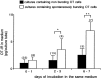
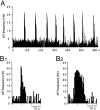
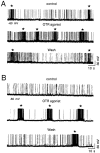
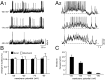
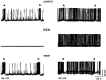
During lactation and parturition, magnocellular oxytocin (OT) neurons display a characteristic bursting electrical activity responsible for pulsatile OT release. We investigated this activity using hypothalamic organotypic slice cultures enriched in magnocellular OT neurons. As shown here, the neurons are functional and actively secrete amidated OT into the cultures. Intracellular recordings were made from 23 spontaneously bursting and 28 slow irregular neurons, all identified as oxytocinergic with biocytin and immunocytochemistry. The bursting electrical activity was similar to that described in vivo and was characterized by bursts of action potentials (20.1 +/- 4.3 Hz) lasting approximately 6 sec, over an irregular background activity. OT (0.1-1 microM), added to the medium, increased burst frequency, reducing interburst intervals by 70%. The peptide also triggered bursting in 27% of nonbursting neurons. These effects were mimicked by the oxytocin receptor (OTR) agonist [Thr4, Gly7]-OT and inhibited by the OTR antagonist desGly-NH2d(CH2)5[D-Tyr2,Thr4]OVT. Burst rhythmicity was independent of membrane potential. Hyperpolarization of the cells unmasked volleys of afferent EPSPs underlying the bursts, which were blocked by CNQX, an AMPA/kainate receptor antagonist. Our results reveal that OT neurons are part of a hypothalamic rhythmic network in which a glutamatergic input governs burst generation. OT neurons, in turn, exert a positive feedback on their afferent drive through the release of OT.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
