Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors
- PMID: 9712650
- PMCID: PMC6792983
- DOI: 10.1523/JNEUROSCI.18-17-06790.1998
Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors
Abstract
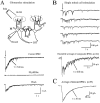
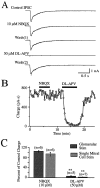
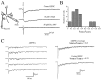
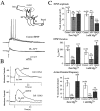
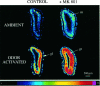
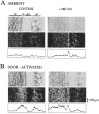
At many central excitatory synapses, AMPA receptors relay the electrical signal, whereas activation of NMDA receptors is conditional and serves a modulatory function. We show here quite a different role for NMDA receptors at dendrodendritic synapses between mitral and granule cells in the rat olfactory bulb. In whole-cell patch-clamp recordings in bulb slices, stimulation of mitral cells elicited slowly decaying, GABAA receptor-mediated reciprocal IPSCs that reflected prolonged GABA release from granule cells. Although granule cells had a normal complement of AMPA and NMDA receptors, the IPSC was completely blocked by the NMDA receptor antagonist D,L-AP-5, suggesting that NMDA receptor activation is an absolute requirement for dendrodendritic inhibition. The AMPA receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2, 3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX) had no effect on IPSCs in the absence of extracellular magnesium but modestly reduced IPSCs in 1 mM magnesium, indicating that the primary effect of the AMPA receptor-mediated depolarization was to facilitate the unblocking of NMDA receptors. Granule cell voltage recordings indicated that effective spike stimulation in granule cells depended on the slow NMDA receptor kinetics. Granule cells also showed a pronounced delay between synaptic stimulation and action potential generation, suggesting that their intrinsic membrane properties underlie the ineffectiveness of brief AMPA receptor-mediated EPSPs. NMDA receptors also seem to have a central role in dendrodendritic inhibition in vivo, because intraperitoneal dizocilpine maleate (MK-801) injection in young adult rats resulted in disinhibition of mitral cells as measured by the generation of c-fos mRNA. The unique dependence of dendrodendritic inhibition on slow EPSPs generated by NMDA receptors suggests that olfactory information processing depends on long-lasting reciprocal and lateral inhibition.
Figures

References
-
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are colocalized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
-
- Berretta N, Jones RSG. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
