Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells
- PMID: 9712657
- PMCID: PMC6792979
- DOI: 10.1523/JNEUROSCI.18-17-06871.1998
Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells
Abstract
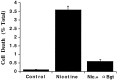
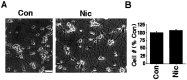
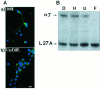
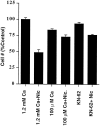
Nicotine has many effects on CNS functions, presumably through its action on neuronal nicotinic acetylcholine receptors (AChRs). One subclass of AChRs that binds the snake venom toxin alpha-bungarotoxin (alpha-Bgt-AChRs) has been shown to modulate neurotransmission in the brain. We now show that alpha-Bgt-AChR activation by low doses of nicotine results in apoptotic cell death of both primary and immortalized hippocampal progenitor cells. In HC2S2-immortalized hippocampal progenitors, nicotine is cytotoxic to undifferentiated cells, whereas it spares the same cells once differentiation has been induced. The activation of alpha-Bgt-AChRs by nicotine results in the induction of the tumor suppressor protein p53 and the cdk inhibitor p21. The cytotoxic effect of nicotine is dependent on extracellular calcium levels and is probably attributable to the poor ability of undifferentiated progenitors to buffer calcium loads. The major calcium buffer in these cells, calbindin D28K, is present only after differentiation has been induced. Furthermore transfection of undifferentiated cells with calbindin results in dramatic protection against the cytotoxic effects of nicotine. These results show that nicotine abuse could have significant effects on the survival of progenitor populations in the developing and adult brain and also suggest an endogenous role for alpha-Bgt-AChRs in neuronal development and differentiation.
Figures


References
-
- Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. V: Alpha-bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. - PubMed
-
- Arends MJ, Harmon BV, Kerr JFR. Apoptosis: the role of the endonuclease. In: Potten CS, editor. Perspectives on mammalian cell death. Oxford UP; New York: 1990. pp. 229–258.
-
- Arneric SP, Sullivan JP, Decker MW, Brioni JD, Bannon AW, Briggs CA, Donnelly-Roberts D, Radek RJ, Marsh KC, Kyncl J, Williams M, Buccafusco JJ. Alzheimer’s disease and associated disorders, Vol 9, pp 50–61. Lippincott–Raven; Philadelphia: 1995. Potential treatment of Alzheimer’s disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolyric-like and cytoprotective properties. - PubMed
-
- Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S. α-Bungarotoxin binding sites in rat hippocampal and cortical cultures: colocalization with α7 subunits and up-regulation by chronic nicotine treatment. Brain Res. 1995;672:228–236. - PubMed
-
- Beck KD, Hefti F, Widmer HR. Deafferentation removes calretinin immunopositive terminals, but does not induce degeneration of calbindin D-28k and parvalbumin expressing neurons in the hippocampus of adult rats. J Neurosci Res. 1994;39:298–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
