Thrombin perturbs neurite outgrowth and induces apoptotic cell death in enriched chick spinal motoneuron cultures through caspase activation
- PMID: 9712658
- PMCID: PMC6792988
- DOI: 10.1523/JNEUROSCI.18-17-06882.1998
Thrombin perturbs neurite outgrowth and induces apoptotic cell death in enriched chick spinal motoneuron cultures through caspase activation
Abstract
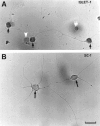
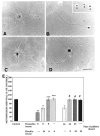
Increasing evidence indicates several roles for thrombin-like serine proteases and their cognate inhibitors (serpins) in normal development and/or pathology of the nervous system. In addition to its prominent role in thrombosis and/or hemostasis, thrombin inhibits neurite outgrowth in neuroblastoma and primary neuronal cells in vitro, prevents stellation of glial cells, and induces cell death in glial and neuronal cell cultures. Thrombin is known to act via a cell surface protease-activated receptor (PAR-1), and recent evidence suggests that rodent neurons express PAR-1. Previously, we have shown that the thrombin inhibitor, protease nexin-1, significantly prevents neuronal cell death both in vitro and in vivo. Here we have examined the effects of human alpha-thrombin and the presence and/or activation of PAR-1 on the survival and differentiation of highly enriched cultures of embryonic chick spinal motoneurons. We show that thrombin significantly decreased the mean neurite length, prevented neurite branching, and induced motoneuron death by an apoptosis-like mechanism in a dose-dependent manner. These effects were prevented by cotreatment with hirudin, a specific thrombin inhibitor. Treatment of the cultures with a synthetic thrombin receptor-activating peptide (SFLLRNP) mimicked the deleterious effects of thrombin on motoneurons. Furthermore, cotreatment of the cultures with inhibitors of caspase activities completely prevented the death of motoneurons induced by either thrombin or SFLLRNP. These findings indicate that (1) embryonic avian spinal motoneurons express functional PAR-1 and (2) activation of this receptor induces neuronal cell degeneration and death via stimulation of caspases. Together with previous reports, our results suggest that thrombin, its receptor(s), and endogenous thrombin inhibitors may be important regulators of neuronal cell fate during development, after injury, and in pathology of the nervous system.
Figures





Similar articles
-
Prevention of thrombin-induced motoneuron degeneration with different neurotrophic factors in highly enriched cultures.J Neurobiol. 1999 Mar;38(4):571-80. J Neurobiol. 1999. PMID: 10084690
-
Activation of the protease-activated thrombin receptor (PAR)-1 induces motoneuron degeneration in the developing avian embryo.J Neuropathol Exp Neurol. 1999 May;58(5):499-504. doi: 10.1097/00005072-199905000-00009. J Neuropathol Exp Neurol. 1999. PMID: 10331438
-
Thrombin induced inhibition of neurite outgrowth from dorsal root ganglion neurons.Brain Res. 1998 Jun 29;797(2):321-7. doi: 10.1016/s0006-8993(98)00344-8. Brain Res. 1998. PMID: 9666159
-
The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system.Brain Res Brain Res Rev. 1997 Sep 30;25(1):85-95. doi: 10.1016/s0165-0173(97)00015-5. Brain Res Brain Res Rev. 1997. PMID: 9370052 Review.
-
Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1.J Cell Biochem. 1989 Jan;39(1):55-64. doi: 10.1002/jcb.240390107. J Cell Biochem. 1989. PMID: 2654147 Review.
Cited by
-
Thrombin mediates migration of rat brain astrocytes via PLC, Ca²⁺, CaMKII, PKCα, and AP-1-dependent matrix metalloproteinase-9 expression.Mol Neurobiol. 2013 Dec;48(3):616-30. doi: 10.1007/s12035-013-8450-6. Epub 2013 Apr 13. Mol Neurobiol. 2013. PMID: 23585120
-
Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis.J Cell Biol. 2000 Sep 4;150(5):1177-88. doi: 10.1083/jcb.150.5.1177. J Cell Biol. 2000. PMID: 10974004 Free PMC article.
-
Thrombin inhibits Bim (Bcl-2-interacting mediator of cell death) expression and prevents serum-withdrawal-induced apoptosis via protease-activated receptor 1.Biochem J. 2003 Oct 1;375(Pt 1):99-109. doi: 10.1042/BJ20030346. Biochem J. 2003. PMID: 12844349 Free PMC article.
-
Plasmin Activation of Glial Cells through Protease-Activated Receptor 1.Patholog Res Int. 2013;2013:314709. doi: 10.1155/2013/314709. Epub 2013 Jan 28. Patholog Res Int. 2013. PMID: 23431500 Free PMC article.
-
Thrombin and the Coag-Inflammatory Nexus in Neurotrauma, ALS, and Other Neurodegenerative Disorders.Front Neurol. 2019 Feb 5;10:59. doi: 10.3389/fneur.2019.00059. eCollection 2019. Front Neurol. 2019. PMID: 30804878 Free PMC article. Review.
References
-
- Abraham CR, Selkoe D, Potter H. Immunochemical identification of the serine protease inhibitor alpha-1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. - PubMed
-
- Baker JB, Low DA, Simmer RL, Cunningham DD. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980;21:37–45. - PubMed
-
- Barinaga M. Forging a path to cell death. Science. 1996;273:735–737. - PubMed
-
- Beecher KL, Anderson TT, Fenton JW, II, Festoff BW. Thrombin receptor peptides induce shape changes in neonatal murine astrocytes in culture. J Neurosci Res. 1994;37:108–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
