Functional magnetic resonance imaging of early visual pathways in dyslexia
- PMID: 9712663
- PMCID: PMC6792964
- DOI: 10.1523/JNEUROSCI.18-17-06939.1998
Functional magnetic resonance imaging of early visual pathways in dyslexia
Abstract
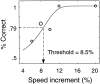
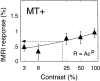
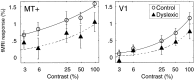
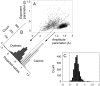
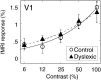
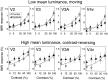
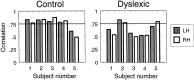
We measured brain activity, perceptual thresholds, and reading performance in a group of dyslexic and normal readers to test the hypothesis that dyslexia is associated with an abnormality in the magnocellular (M) pathway of the early visual system. Functional magnetic resonance imaging (fMRI) was used to measure brain activity in conditions designed to preferentially stimulate the M pathway. Speed discrimination thresholds, which measure the minimal increase in stimulus speed that is just noticeable, were acquired in a paradigm modeled after a previous study of M pathway-lesioned monkeys. Dyslexics showed reduced brain activity compared with controls both in primary visual cortex (V1) and in several extrastriate areas, including area MT and adjacent motion-sensitive areas (MT+) that are believed to receive a predominant M pathway input. There was a strong three-way correlation between brain activity, speed discrimination thresholds, and reading speed. Subjects with higher V1 and MT+ responses had lower perceptual thresholds (better performance) and were faster readers. These results support the hypothesis for an M pathway abnormality in dyslexia and imply strong relationships between the integrity of the M pathway, visual motion perception, and reading ability.
Figures




References
-
- Albrecht DG. Visual cortex neurons in monkey and cat: effect of contrast on the spatial and temporal phase transfer functions. Vis Neurosci. 1995;12:1191–1210. - PubMed
-
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;78:516–520. - PubMed
-
- Beckers G, Homberg V. Cerebral visual motion blindness: transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proc R Soc Lond B Biol Sci. 1992;249:173–178. - PubMed
-
- Beckers G, Zeki S. The consequences of inactivating areas V1 and V5 on visual motion perception. Brain. 1995;118:49–60. - PubMed
-
- Borsting E, Ridder WH, Dudeck K, Kelley C, Matsui L, Motoyama J. The presence of a magnocellular defect depends on the type of dyslexia. Vision Res. 1996;36:1047–1053. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
