Nitric oxide signaling in pain and nociceptor sensitization in the rat
- PMID: 9712669
- PMCID: PMC6792985
- DOI: 10.1523/JNEUROSCI.18-17-07008.1998
Nitric oxide signaling in pain and nociceptor sensitization in the rat
Abstract
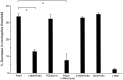
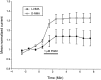
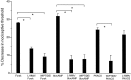
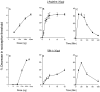
We investigated the role of nitric oxide (NO) in inflammatory hyperalgesia. Coinjection of prostaglandin E2 (PGE2) with the nitric oxide synthase (NOS) inhibitor NG-methyl-L-arginine (L-NMA) inhibited PGE2-induced hyperalgesia. L-NMA was also able to reverse that hyperalgesia. This suggests that NO contributes to the maintenance of, as well as to the induction of, PGE2-induced hyperalgesia. Consistent with the hypothesis that the NO that contributes to PGE2-induced sensitization of primary afferents is generated in the dorsal root ganglion (DRG) neurons themselves, L-NMA also inhibited the PGE2-induced increase in tetrodotoxin-resistant sodium current in patch-clamp electrophysiological studies of small diameter DRG neurons in vitro. Although NO, the product of NOS, often activates guanylyl cyclase, we found that PGE2-induced hyperalgesia was not inhibited by coinjection of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a guanylyl cyclase inhibitor. We then tested whether the effect of NO depended on interaction with the adenylyl cyclase-protein kinase A (PKA) pathway, which is known to mediate PGE2-induced hyperalgesia. L-NMA inhibited hyperalgesia produced by 8-bromo-cAMP (a stable membrane permeable analog of cAMP) or by forskolin (an adenylyl cyclase activator). However, L-NMA did not inhibit hyperalgesia produced by injection of the catalytic subunit of PKA. Therefore, the contribution of NO to PGE2-induced hyperalgesia may occur in the cAMP second messenger pathway at a point before the action of PKA. We next performed experiments to test whether administration of exogenous NO precursor or donor could mimic the hyperalgesic effect of endogenous NO. Intradermal injection of either the NOS substrate L-arginine or the NO donor 3-(4-morphinolinyl)-sydnonimine hydrochloride (SIN-1) produced hyperalgesia. However, this hyperalgesia differed from PGE2-induced hyperalgesia, because it was independent of the cAMP second messenger system and blocked by the guanylyl cyclase inhibitor ODQ. Therefore, although exogenous NO induces hyperalgesia, it acts by a mechanism different from that by which endogenous NO facilitates PGE2-induced hyperalgesia. Consistent with the hypothesis that these mechanisms are distinct, we found that inhibition of PGE2-induced hyperalgesia caused by L-NMA could be reversed by a low dose of the NO donor SIN-1. The following facts suggest that this dose of SIN-1 mimics a permissive effect of basal levels of NO with regard to PGE2-induced hyperalgesia: (1) this dose of SIN-1 does not produce hyperalgesia when administered alone, and (2) the effect was not blocked by ODQ. In conclusion, we have shown that low levels of NO facilitate cAMP-dependent PGE2-induced hyperalgesia, whereas higher levels of NO produce a cGMP-dependent hyperalgesia.
Figures


References
-
- Beesley JE. Histochemical methods for detecting nitric oxide synthase. Histochem J. 1995;27:757–769. - PubMed
-
- Burgstahler AD, Nathanson MH. NO modulates the apicolateral cytoskeleton of isolated hepatocytes by a PKC-dependent, cGMP-independent mechanism. Am J Physiol. 1995;269:G789–G799. - PubMed
-
- Choi Y, Raja SN, Moore LC, Tobin JR. Neuropathic pain in rats is associated with altered nitric oxide synthase activity in neural tissue. J Neurol Sci. 1996;138:14–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
