Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori
- PMID: 9712748
- PMCID: PMC108486
- DOI: 10.1128/IAI.66.9.4061-4067.1998
Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori
Abstract
The in vitro glycolipid binding specificity of the gastric pathogen Helicobacter pylori is altered to include sulfated glycolipids (sulfatides) following brief exposure of the organism to acid pH typical of the stomach. This change is prevented by anti-hsp70 antibodies, suggesting that hsp70 may be a stress-induced surface adhesin, mediating sulfatide recognition. To facilitate investigation of the role of hsp70 in attachment, we have cloned and sequenced the H. pylori hsp70 gene (dnaK). The hsp70 gene was identified by probing a cosmid DNA library made from H. pylori 439 with a PCR amplicon generated with oligonucleotides synthesized to highly conserved regions of dnaK. The 1.9-kb H. pylori hsp70 gene encodes a product of 616 amino acids. Primer extension analysis revealed a single transcription start site, while Northern blot analysis established that hsp70 was preferentially induced by low pH rather than by heat shock. The ability of H. pylori to alter its glycolipid binding specificity following exposure to low pH by upregulating hsp70 and by expressing hsp70 on the bacterial surface may provide a survival advantage during periods of high acid stress.
Figures



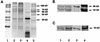
References
-
- Borén T, Falk P, Roth K A, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. - PubMed
-
- Boulanger J, Faulds D, Eddy E M, Lingwood C A. Members of the 70kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma related infertility. J Cell Physiol. 1995;165:7–17. - PubMed
-
- Boulanger J, Huesca M, Arab S, Lingwood C A. Universal method for the facile production of glycolipid/lipid matrices for the affinity purification of binding ligands. Anal Biochem. 1994;217:1–6. - PubMed
-
- Busse J, Hartmann E, Lingwood C A. Receptor affinity purification of a lipid-binding adhesin from Haemophilus influenzae. J Infect Dis. 1996;175:77–83. - PubMed
-
- Cave D R, Vargal M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet. 1989;ii:187–189. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

