Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis
- PMID: 9712769
- PMCID: PMC108507
- DOI: 10.1128/IAI.66.9.4208-4214.1998
Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis
Abstract
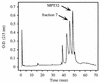
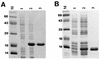
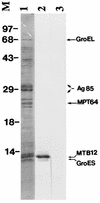
Proteins secreted into the culture medium by Mycobacterium tuberculosis are thought to play an important role in the development of protective immune responses. In this report, we describe the molecular cloning of a novel, low-molecular-weight antigen (MTB12) secreted by M. tuberculosis. Sequence analysis of the MTB12 gene indicates that the protein is initially synthesized as a 16.6-kDa precursor protein containing a 48-amino-acid hydrophobic leader sequence. The mature, fully processed form of MTB12 protein found in culture filtrates has a molecular mass of 12. 5 kDa. MTB12 protein constitutes a major component of the M. tuberculosis culture supernatant and appears to be at least as abundant as several other well-characterized culture filtrate proteins, including members of the 85B complex. MTB12 is encoded by a single-copy gene which is present in both virulent and avirulent strains of the M. tuberculosis complex, the BCG strain of M. bovis, and M. leprae. Recombinant MTB12 containing an N-terminal six-histidine tag was expressed in Escherichia coli and purified by affinity chromatography. Recombinant MTB12 protein elicited in vitro proliferative responses from the peripheral blood mononuclear cells of a number of purified protein derivative-positive (PPD+) human donors but not from PPD- donors.
Figures

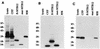
References
-
- Aldrich C J, De Cloux A, Woods A S, Cotter R J, Soloski M J, Forman J. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell. 1994;79:649–658. - PubMed
-
- Braud V, Jones E Y, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

