Coiling phagocytosis of trypanosomatids and fungal cells
- PMID: 9712785
- PMCID: PMC108523
- DOI: 10.1128/IAI.66.9.4331-4339.1998
Coiling phagocytosis of trypanosomatids and fungal cells
Abstract
Coiling phagocytosis has previously been studied only with the bacteria Legionella pneumophila and Borrelia burgdorferi, and the results were inconsistent. To learn more about this unconventional phagocytic mechanism, the uptake of various eukaryotic microorganisms by human monocytes, murine macrophages, and murine dendritic cells was investigated in vitro by video and electron microscopy. Unconventional phagocytosis of Leishmania spp. promastigotes, Trypanosoma cruzi trypomastigotes, Candida albicans hyphae, and zymosan particles from Saccharomyces cerevisiae differed in (i) morphology (rotating unilateral pseudopods with the trypanosomatids, overlapping bilateral pseudopods with the fungi), (ii) frequency (high with Leishmania; occasional with the fungi; rare with T. cruzi), (iii) duration (rapid with zymosan; moderate with the trypanosomatids; slow with C. albicans), (iv) localization along the promastigotes (flagellum of Leishmania major and L. aethiopica; flagellum or posterior pole of L. donovani), and (v) dependence on complement (strong with L. major and L. donovani; moderate with the fungi; none with L. aethiopica). All of these various types of unconventional phagocytosis gave rise to similar pseudopod stacks which eventually transformed to a regular phagosome. Further video microscopic studies with L. major provided evidence for a cytosolic localization, synchronized replication, and exocytic release of the parasites, extending traditional concepts about leishmanial infection of host cells. It is concluded that coiling phagocytosis comprises phenotypically similar consequences of various disturbances in conventional phagocytosis rather than representing a single separate mechanism.
Figures
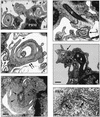
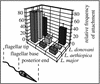
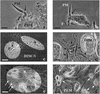



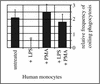
Similar articles
-
In vivo and in vitro phagocytosis of Leishmania (Leishmania) amazonensis promastigotes by B-1 cells.Parasite Immunol. 2016 Jun;38(6):365-76. doi: 10.1111/pim.12324. Parasite Immunol. 2016. PMID: 27084328
-
Phagocytes from both vertebrate and invertebrate species use "coiling" phagocytosis.Dev Comp Immunol. 1996 Nov-Dec;20(6):393-406. doi: 10.1016/s0145-305x(96)00023-7. Dev Comp Immunol. 1996. PMID: 9040982
-
The effects of macrophage source on the mechanism of phagocytosis and intracellular survival of Leishmania.Microbes Infect. 2011 Nov;13(12-13):1033-44. doi: 10.1016/j.micinf.2011.05.014. Epub 2011 Jun 30. Microbes Infect. 2011. PMID: 21723411 Free PMC article.
-
Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand?Microbes Infect. 1999 Jul;1(9):727-35. doi: 10.1016/s1286-4579(99)80074-4. Microbes Infect. 1999. PMID: 10611750 Review.
-
Intracellular mechanisms of killing.Contemp Top Immunobiol. 1984;12:53-69. doi: 10.1007/978-1-4684-4571-8_2. Contemp Top Immunobiol. 1984. PMID: 6365446 Review. No abstract available.
Cited by
-
Dendritic cells phagocytose and are activated by Treponema pallidum.Infect Immun. 2001 Jan;69(1):518-28. doi: 10.1128/IAI.69.1.518-528.2001. Infect Immun. 2001. PMID: 11119545 Free PMC article.
-
Leishmania mexicana pathogenicity requires flagellar assembly but not motility.Virulence. 2025 Dec;16(1):2521478. doi: 10.1080/21505594.2025.2521478. Epub 2025 Jul 2. Virulence. 2025. PMID: 40602995 Free PMC article.
-
Trypanosoma cruzi: Entry into Mammalian Host Cells and Parasitophorous Vacuole Formation.Front Immunol. 2013 Aug 1;4:186. doi: 10.3389/fimmu.2013.00186. eCollection 2013. Front Immunol. 2013. PMID: 23914186 Free PMC article.
-
Differentiating Trypanosoma cruzi in a Host Mammalian Cell Imaged in Aqueous Liquid by Atmospheric Scanning Electron Microscopy.Microbiol Spectr. 2022 Feb 23;10(1):e0141321. doi: 10.1128/spectrum.01413-21. Epub 2022 Jan 5. Microbiol Spectr. 2022. PMID: 34985339 Free PMC article.
-
Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding.Open Biol. 2017 Sep;7(9):170165. doi: 10.1098/rsob.170165. Open Biol. 2017. PMID: 28903998 Free PMC article. Review.
References
-
- Akiyama H J, Haight R D. Interaction of Leishmania donovani and hamster peritoneal macrophages. A phase-contrast microscopical study. Am J Trop Med Hyg. 1971;20:539–545. - PubMed
-
- Akuffo H, Maasho K, Blostedt M, Höjeberg B, Britton S, Bakhiet M. Leishmania aethiopica derived from diffuse leishmaniosis patients preferentially induce mRNA for interleukin-12 while those from localized leishmaniosis patients induce interferon-γ. J Infect Dis. 1997;175:737–741. - PubMed
-
- Behrendt H, Seemayer N H, Braumann A, Nissen M. Electron microscopy investigations of the effect of quartz dust DQ 12 on human monocytes/macrophages in vitro. Silikoserep Nordrhein-Westphalen. 1987;16:171–183.
-
- Beverley S M, Turco S J. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

