Regeneration and transplantation of the optic nerve: developing a clinical strategy
- PMID: 9713068
- PMCID: PMC1722609
- DOI: 10.1136/bjo.82.5.577
Regeneration and transplantation of the optic nerve: developing a clinical strategy
Abstract
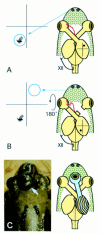
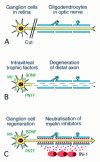
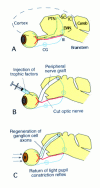
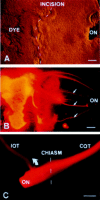
Three separate experimental models of optic nerve regeneration have been presented--along the existing pathway in the presence of antibodies to neutralise inhibitory molecules, along peripheral nerve grafts and from retinal transplants. Each offers a theoretical clinical strategy for restoration of vision, if the mechanism of re-establishment of maps and reconnection to appropriate targets during regeneration can be determined. This is the process of axon guidance, and underlines the importance of our research into the molecular determinants that guide normal development of the visual system.
Figures

Similar articles
-
Regeneration of rat optic axons into peripheral nerve grafts.Exp Neurol. 1986 Jan;91(1):52-9. doi: 10.1016/0014-4886(86)90025-7. Exp Neurol. 1986. PMID: 3940879
-
Regeneration in the optic nerve of adult rats: influences of cultured astrocytes and optic nerve grafts of different ontogenetic stages.J Neurocytol. 1995 Oct;24(10):783-93. doi: 10.1007/BF01191214. J Neurocytol. 1995. PMID: 8586998
-
Advances in experimental optic nerve regeneration.Curr Opin Ophthalmol. 2017 Nov;28(6):558-563. doi: 10.1097/ICU.0000000000000417. Curr Opin Ophthalmol. 2017. PMID: 28795960 Review.
-
[Optic nerve regeneration by nerve transplantation].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):956-71. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022308 Review. Japanese.
-
[Optic nerve regeneration and functional recovery of vision following peripheral nerve transplant].No To Shinkei. 1998 Mar;50(3):227-35. No To Shinkei. 1998. PMID: 9565997 Review. Japanese. No abstract available.
Cited by
-
Emerging options for the management of age-related macular degeneration with stem cells.Stem Cells Cloning. 2010 Dec 22;4:1-10. doi: 10.2147/SCCAA.S7674. Stem Cells Cloning. 2010. PMID: 24198525 Free PMC article. Review.
-
Impediments to eye transplantation: ocular viability following optic-nerve transection or enucleation.Br J Ophthalmol. 2009 Sep;93(9):1134-40. doi: 10.1136/bjo.2008.155267. Epub 2009 Mar 13. Br J Ophthalmol. 2009. PMID: 19286686 Free PMC article. Review.
-
Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation).Neural Regen Res. 2016 Jun;11(6):1006-12. doi: 10.4103/1673-5374.184505. Neural Regen Res. 2016. PMID: 27482233 Free PMC article.
-
Early decompression of the injured optic nerve reduces axonal degeneration and improves functional outcome in the adult rat.Exp Brain Res. 2007 May;179(1):121-30. doi: 10.1007/s00221-006-0775-1. Epub 2006 Nov 14. Exp Brain Res. 2007. PMID: 17103208
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
