Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia
- PMID: 9714859
- PMCID: PMC2231151
- DOI: 10.1111/j.1469-7793.1998.771bg.x
Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia
Abstract
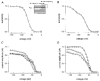
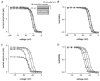
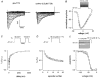
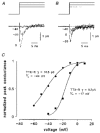
1. Whole-cell and single-channel Na+ currents were recorded from small (ca. 20 micron diameter) cells isolated from adult rat dorsal root ganglia (DRG). Currents were classified by their sensitivity to 0.3 microM tetrodotoxin (TTX), electrophysiological properties and single-channel amplitude. Cells were classified according to the types of current recorded from them. 2. Type A cells expressed essentially pure TTX-sensitive (TTX-S) currents. Availability experiments with prepulse durations between 50 ms and 1 s gave a half-available voltage (Vh) of around -65 mV but the availability curves often had a complex shape, consistent with multiple inactivation processes. Measured inactivation time constants ranged from less than 1 ms to over 100 s, depending on the protocol used. 3. Cell types B and C each had, in addition to TTX-S currents, substantial and different TTX-resistant (TTX-R) currents that we have designated TTX-R1 and TTX-R2, respectively. TTX-R1 currents had a 1 s Vh of -29 mV, showed little 1 Hz use dependence at -67 mV and recovered from the inactivation induced by a 60 ms depolarizing pulse with time constants of 1.6 ms (91 %) and 908 ms. They also exhibited slow inactivation processes with component time constants around 10 and 100 s. TTX-R2 currents activated and inactivated at more negative potentials (1 s Vh = -46 mV), showed substantial 1 Hz use dependence and had inactivation (60 ms pulse) recovery time constants at -67 mV of 3.3 ms (58 %) and 902 ms. 4. Type D cells had little or no current in 0.3 microM TTX at a holding potential of -67 mV. Current amplitude increased on changing the holding potential to -107 mV. Type D cell currents had more hyperpolarized availability and I-V curves than even TTX-R2 currents and suggest the existence of TTX-R3 channels. 5. In outside-out patches with 250 mM external NaCl, the single-channel conductance (gamma) of TTX-S channels was 19.5 pS and the potential for half-maximal activation (Va) was -45 mV. One population of TTX-R channels had a gamma of 9.2 pS and a Va of -27 mV. A second population had a gamma of 16.5 pS and a more negative Va of -42 mV. The latter population may underlie the type D cell current. 6. Small DRG cells express multiple Na+ currents with varied time constants and voltage dependences of activation and inactivation. Nociceptive cells still fire when chronically depolarized by an increased external K+ concentration. TTX-R1 and TTX-R2 Na+ channels may support that firing, while the range of inactivation time constants described here would increase the repertoire of DRG cell burst firing behaviour generally.
Figures




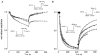
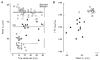



References
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Molecular Brain Research. 1996;43:117–131. - PubMed
-
- Bräu ME, Rush AM, Elliott JR. Single channel analysis of two types of tetrodotoxin-resistant sodium channels (TTX-r INa) in small cells isolated from adult rat (200-350 g) dorsal root ganglia (DRG) The Journal of Physiology. 1995;487.P:184. P.
-
- Buchanan S, Harper AA, Elliott JR. Differential effects of tetrodotoxin (TTX) and high external K+ on A and C fibre compound action potential peaks in frog sciatic nerve. Neuroscience Letters. 1996;219:131–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

