Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways
- PMID: 9716412
- PMCID: PMC317102
- DOI: 10.1101/gad.12.16.2610
Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways
Abstract
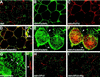
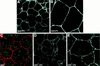
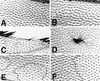
In Drosophila, planar cell polarity (PCP) signaling is mediated by the receptor Frizzled (Fz) and transduced by Dishevelled (Dsh). Wingless (Wg) signaling also requires Dsh and may utilize DFz2 as a receptor. Using a heterologous system, we show that Dsh is recruited selectively to the membrane by Fz but not DFz2, and this recruitment depends on the DEP domain but not the PDZ domain in Dsh. A mutation in the DEP domain impairs both membrane localization and the function of Dsh in PCP signaling, indicating that translocation is important for function. Further genetic and molecular analyses suggest that conserved domains in Dsh function differently during PCP and Wg signaling, and that divergent intracellular pathways are activated. We propose that Dsh has distinct roles in PCP and Wg signaling. The PCP signal may selectively result in focal Fz activation and asymmetric relocalization of Dsh to the membrane, where Dsh effects cytoskeletal reorganization to orient prehair initiation.
Figures

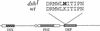


References
-
- Adler PN. The genetic control of tissue polarity in Drosophila. BioEssays. 1992;14:735–741. - PubMed
-
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. - PubMed
-
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Dishevelled mediates interaction between Wingless and Notch signaling pathways. Science. 1996;271:1826–1832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
