Biochemistry and regulation of a novel Escherichia coli K-12 porin protein, OmpG, which produces unusually large channels
- PMID: 9721282
- PMCID: PMC107454
- DOI: 10.1128/JB.180.17.4452-4459.1998
Biochemistry and regulation of a novel Escherichia coli K-12 porin protein, OmpG, which produces unusually large channels
Abstract
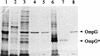
A novel porin, OmpG, is produced in response to a chromosomal mutation termed cog-192. Molecular characterization of cog-192 revealed that it is a large chromosomal deletion extending from the 3' end of pspA through to the 5' end of an open reading frame located immediately upstream of ompG. As a result of this 13.1-kb deletion, the expression of ompG was placed under the control of the pspA promoter. Characterization of OmpG revealed that it is quite different from other porins. Proteoliposome swelling assays showed that OmpG channels were much larger than those of the OmpF and OmpC porins, with an estimated limited diameter of about 2 nm. The channel lacked any obvious solute specificity. The folding model of OmpG suggests that it is the first 16-stranded beta-barrel porin that lacks the large external loop, L3, which constricts the channels of other nonspecific and specific porins. Consistent with the folding model, circular dichroism showed that OmpG contains largely a beta-sheet structure. In contrast to other Escherichia coli porins, there is no evidence that OmpG exists as stable oligomers. Although ompG DNA was present in all E. coli strains examined so far, its expression under laboratory conditions was seen only due to rare chromosomal mutations. Curiously, OmpG was constitutively expressed, albeit at low levels, in Salmonella, Shigella, and Pseudomonas species.
Figures






References
-
- Ames B N, Hartman P E, Jacob F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes of Salmonella. J Mol Biol. 1963;7:23–42. - PubMed
-
- Benson S A, Occi J L, Sampson B A. Mutations that alter the pore function of the OmpF porin of Escherichia coli K-12. J Mol Biol. 1988;203:961–970. - PubMed
-
- Benz R. Uptake of solutes through bacterial outer membranes. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 397–423.
-
- Blasband A J, Marcotte W R, Jr, Schnaitman C A. Structure of the lc and nmpC outer membrane porin protein genes of lambdoid bacteriophage. J Mol Biol. 1986;261:12723–12732. - PubMed
-
- Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

