Molecular characterization and sequence of a methionine biosynthetic locus from Pseudomonas syringae
- PMID: 9721288
- PMCID: PMC107460
- DOI: 10.1128/JB.180.17.4497-4507.1998
Molecular characterization and sequence of a methionine biosynthetic locus from Pseudomonas syringae
Abstract
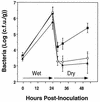
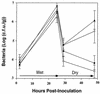
Two methionine biosynthetic genes in Pseudomonas syringae pv. syringae, metX and metW, were isolated, sequenced, and evaluated for their roles in methionine biosynthesis and bacterial fitness on leaf surfaces. The metXW locus was isolated on a 1.8-kb DNA fragment that was required for both methionine prototrophy and wild-type epiphytic fitness. Sequence analysis identified two consecutive open reading frames (ORFs), and in vitro transcription-translation experiments provided strong evidence that the ORFs encode proteins with the predicted molecular masses of 39 and 22.5 kDa. The predicted amino acid sequence of MetX (39 kDa) showed homology to several known and putative homoserine O-acetyltransferases. This enzyme is the first enzyme in the methionine biosynthetic pathway of fungi, gram-negative bacteria of the genus Leptospira, and several gram-positive bacterial genera. Both metX and metW were required for methionine biosynthesis, and transcription from both genes was not repressed by methionine. MetW (22.5 kDa) did not show significant homology to any known protein, including prokaryotic and eukaryotic methionine biosynthetic enzymes. Several classes of methionine auxotrophs, including metX and metW mutants, exhibit reduced fitness on leaf surfaces, indicating a requirement for methionine prototrophy in wild-type epiphytic fitness. This requirement is enhanced under environmentally stressful conditions, suggesting a role for methionine prototrophy in bacterial stress tolerance.
Figures
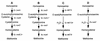





References
-
- Anderson D M, Mills D. The use of transposon mutagenesis in the isolation of nutritional and virulent mutants in two pathovars of Pseudomonas syringae. Phytopathology. 1985;75:104–108.
-
- Baroni M, Livian S, Martegani E, Alberghina L. Molecular cloning and regulation of the expression of the MET2 gene of Saccharomyces cerevisiae. Gene. 1986;46:71–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

