Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores
- PMID: 9721302
- PMCID: PMC107474
- DOI: 10.1128/JB.180.17.4603-4612.1998
Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores
Abstract
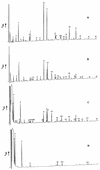
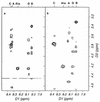
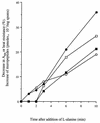
Peptidoglycan structural dynamics during endospore germination of Bacillus subtilis 168 have been examined by muropeptide analysis. The first germination-associated peptidoglycan structural changes are detected within 3 min after the addition of the specific germinant L-alanine. We detected in the spore-associated material new muropeptides which, although they have slightly longer retention times by reversed-phase (RP)-high-pressure liquid chromatography (HPLC) than related ones in dormant spores, show the same amino acid composition and molecular mass. Two-dimensional nuclear magnetic resonance (NMR) analysis shows that the chemical changes to the muropeptides on germination are minor and are probably limited to stereochemical inversion. These new muropeptides account for almost 26% of the total muropeptides in spore-associated material after 2 h of germination. The exudate of germinated spores of B. subtilis 168 contains novel muropeptides in addition to those present in spore-associated material. Exudate-specific muropeptides have longer retention times, have no reducing termini, and exhibit a molecular mass 20 Da lower than those of related reduced muropeptides. These new products are anhydro-muropeptides which are generated by a lytic transglycosylase, the first to be identified in a gram-positive bacterium. There is also evidence for the activity of a glucosaminidase during the germination process. Quantification of muropeptides in spore-associated material indicates that there is a heterogeneous distribution of muropeptides in spore peptidoglycan. The spore-specific residue, muramic delta-lactam, is proposed to be a major substrate specificity determinant of germination-specific lytic enzymes, allowing cortex hydrolysis without any effect on the primordial cell wall.
Figures


References
-
- Brown K L. Spore resistance and ultra heat treatment processes. J Appl Bacteriol. 1994;76:67S–80S. - PubMed
-
- Cleveland E F, Gilvarg C. Selective degradation of peptidoglycan from Bacillus megaterium spores during germination. In: Gerhardt P, Costilow R N, Sadoff H L, editors. Spores VI. Washington, D.C: American Society for Microbiology; 1975. pp. 458–464.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

