The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain
- PMID: 9722603
- PMCID: PMC2132874
- DOI: 10.1083/jcb.142.4.887
The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain
Abstract
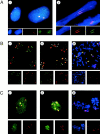
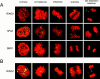
The Polycomb group (PcG) complex is a chromatin-associated multiprotein complex, involved in the stable repression of homeotic gene activity in Drosophila. Recently, a mammalian PcG complex has been identified with several PcG proteins implicated in the regulation of Hox gene expression. Although the mammalian PcG complex appears analogous to the complex in Drosophila, the molecular mechanisms and functions for the mammalian PcG complex remain unknown. Here we describe a detailed characterization of the human PcG complex in terms of cellular localization and chromosomal association. By using antibodies that specifically recognize three human PcG proteins- RING1, BMI1, and hPc2-we demonstrate in a number of human cell lines that the PcG complex forms a unique discrete nuclear structure that we term PcG bodies. PcG bodies are prominent novel nuclear structures with the larger PcG foci generally localized near the centromeres, as visualized with a kinetochore antibody marker. In both normal fetal and adult fibroblasts, PcG bodies are not randomly dispersed, but appear clustered into defined areas within the nucleus. We show in three different human cell lines that the PcG complex can tightly associate with large pericentromeric heterochromatin regions (1q12) on chromosome 1, and with related pericentromeric sequences on different chromosomes, providing evidence for a mammalian PcG-heterochromatin association. Furthermore, these heterochromatin-bound PcG complexes remain stably associated throughout mitosis, thereby allowing the potential inheritance of the PcG complex through successive cell divisions. We discuss these results in terms of the known function of the PcG complex as a transcriptional repression complex.
Figures





References
-
- Alkema MJ, Bronk M, Verhoeven E, Otte A, van't Veer LJ, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian Polycomb complex. Genes Dev. 1997;11:226–240. - PubMed
-
- Bienz M, Müller J. Transcriptional silencing of homeotic genes in Drosophila. . Bioessays. 1995;17:775–784. - PubMed
-
- Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

