Differential modulation of SERCA2 isoforms by calreticulin
- PMID: 9722609
- PMCID: PMC2132884
- DOI: 10.1083/jcb.142.4.963
Differential modulation of SERCA2 isoforms by calreticulin
Abstract
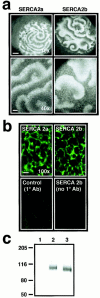
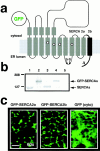
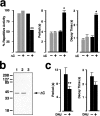
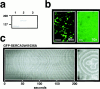
In Xenopus laevis oocytes, overexpression of calreticulin suppresses inositol 1,4,5-trisphosphate-induced Ca2+ oscillations in a manner consistent with inhibition of Ca2+ uptake into the endoplasmic reticulum. Here we report that the alternatively spliced isoforms of the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA)2 gene display differential Ca2+ wave properties and sensitivity to modulation by calreticulin. We demonstrate by glucosidase inhibition and site-directed mutagenesis that a putative glycosylated residue (N1036) in SERCA2b is critical in determining both the selective targeting of calreticulin to SERCA2b and isoform functional differences. Calreticulin belongs to a novel class of lectin ER chaperones that modulate immature protein folding. In addition to this role, we suggest that these chaperones dynamically modulate the conformation of mature glycoproteins, thereby affecting their function.
Figures



References
-
- Baksh S, Burns K, Andrin C, Michalak M. Interaction of calreticulin with protein disulfide isomerase. J Biol Chem. 1995;270:31338–31344. - PubMed
-
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+stores and reveals aspects of their luminal microenvironment and function. J Cell Biol. 1995;130:847–855. - PMC - PubMed
-
- Bayle D, Weeks D, Sachs G. The membrane topology of the rat sarcoplasmic and endoplasmic reticulum calcium ATPases by in vitro translation scanning. J Biol Chem. 1995;270:25678–25684. - PubMed
-
- Bergeron JJM, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

