Directed expression of keratin 16 to the progenitor basal cells of transgenic mouse skin delays skin maturation
- PMID: 9722615
- PMCID: PMC2132878
- DOI: 10.1083/jcb.142.4.1035
Directed expression of keratin 16 to the progenitor basal cells of transgenic mouse skin delays skin maturation
Abstract
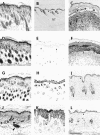
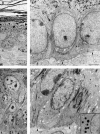
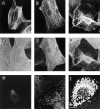
We previously hypothesized that the type I keratin 16 (K16) plays a role in the process of keratinocyte activation that occurs in response to skin injury (Paladini, R.D., K. Takahashi, N.S. Bravo, and P.A. Coulombe. 1996. J. Cell Biol. 132:381-397). To further examine its properties in vivo, the human K16 cDNA was constitutively expressed in the progenitor basal layer of transgenic mouse skin using the K14 gene promoter. Mice that express approximately as much K16 protein as endogenous K14 display a dramatic postnatal phenotype that consists of skin that is hyperkeratotic, scaly, and essentially devoid of fur. Histologically, the epidermis is thickened because of hyperproliferation of transgenic basal cells, whereas the hair follicles are decreased in number, poorly developed, and hypoproliferative. Microscopically, the transgenic keratinocytes are hypertrophic and feature an altered keratin filament network and decreased cell-cell adhesion. The phenotype normalizes at approximately 5 wk after birth. In contrast, control mice expressing a K16-K14 chimeric protein to comparable levels are normal. The character and temporal evolution of the phenotype in the K16 transgenic mice are reminiscent of the activated EGF receptor- mediated signaling pathway in skin. In fact, tyrosine phosphorylation of the EGF receptor is increased in the newborn skin of K16 transgenic mice. We conclude that expression of K16 can significantly alter the response of skin keratinocytes to signaling cues, a distinctive property likely resulting from its unique COOH-terminal tail domain.
Figures









References
-
- Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T. Delayed assembly of desmosomes in keratinocytes with disrupted classic-cadherin-mediated cell adhesion by a dominant negative mutant. J Invest Dermatol. 1995;104:27–32. - PubMed
-
- Bereiter-Hahn, J. 1986. Epidermal cell migration and wound repair. In Biology of the Integument, Vol. 2: Vertebrates. J. Bereiter-Hahn, A.G. Matoltsy, and K.S. Richards, editors. Springer-Verlag, Berlin. 443–471.
-
- Borum K. Hair patterns and hair succession in the albino mouse. Acta Pathol Microbiol Scand. 1954;34:521–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

