Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein
- PMID: 9722621
- PMCID: PMC2132880
- DOI: 10.1083/jcb.142.4.1105
Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein
Abstract
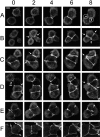
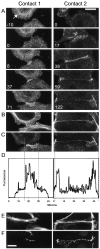
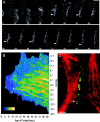
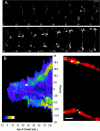
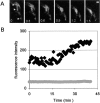
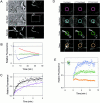
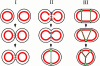
Cadherin-mediated adhesion initiates cell reorganization into tissues, but the mechanisms and dynamics of such adhesion are poorly understood. Using time-lapse imaging and photobleach recovery analyses of a fully functional E-cadherin/GFP fusion protein, we define three sequential stages in cell-cell adhesion and provide evidence for mechanisms involving E-cadherin and the actin cytoskeleton in transitions between these stages. In the first stage, membrane contacts between two cells initiate coalescence of a highly mobile, diffuse pool of cell surface E-cadherin into immobile punctate aggregates along contacting membranes. These E-cadherin aggregates are spatially coincident with membrane attachment sites for actin filaments branching off from circumferential actin cables that circumscribe each cell. In the second stage, circumferential actin cables near cell-cell contact sites separate, and the resulting two ends of the cable swing outwards to the perimeter of the contact. Concomitantly, subsets of E-cadherin puncta are also swept to the margins of the contact where they coalesce into large E-cadherin plaques. This reorganization results in the formation of a circumferential actin cable that circumscribes both cells, and is embedded into each E-cadherin plaque at the contact margin. At this stage, the two cells achieve maximum contact, a process referred to as compaction. These changes in E-cadherin and actin distributions are repeated when additional single cells adhere to large groups of cells. The third stage of adhesion occurs as additional cells are added to groups of >3 cells; circumferential actin cables linked to E-cadherin plaques on adjacent cells appear to constrict in a purse-string action, resulting in the further coalescence of individual plaques into the vertices of multicell contacts. The reorganization of E-cadherin and actin results in the condensation of cells into colonies. We propose a model to explain how, through strengthening and compaction, E-cadherin and actin cables coordinate to remodel initial cell-cell contacts to the final condensation of cells into colonies.
Figures



References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

