Population pharmacokinetics of ondansetron: a covariate analysis
- PMID: 9723819
- PMCID: PMC1873666
- DOI: 10.1046/j.1365-2125.1998.00756.x
Population pharmacokinetics of ondansetron: a covariate analysis
Abstract
Aims: To construct a population model to account for the variability in ondansetron pharmacokinetics and to evaluate methods for the efficient development of population models.
Methods: Population models were developed using 99 subjects consisting of paediatric patients, young, elderly and aged volunteers. A two compartment pharmacokinetic model with a zero order input was used to describe the pharmacokinetics of ondansetron. Three stepwise methods were proposed and used alongside a three step approach to develop population models with both rich and sparse data sets. The stepwise methods were based on obtaining empirical Bayes posterior estimates of pharmacokinetic parameters within a nonlinear mixed effect modelling (NONMEM) program. The parameters were then regressed against covariates in a stepwise procedure. Variance parameters were obtained by fitting the proposed population model to the data in one further NONMEM run. The population model was validated against a test data set of 54 subjects, including children, young and elderly patients and volunteers.
Results: The population model adequately described the differences in ondansetron pharmacokinetics between paediatric patients, young, elderly and aged volunteers. Different covariates were identified by the various methods. Weight was found to have a strong positive linear relationship with all four pharmacokinetic parameters. Clearance showed a weak negative relationship with age. Males were found to have a greater clearance than females after weight adjustment.
Conclusions: The stepwise search procedures potentially are capable of considerably reducing the time required to develop population pharmacokinetic models. The model developed for ondansetron gave accurate predictions of both the concentration-time profile and variability in an independent data set.
Figures

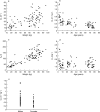

 ) method estimates (based on one run in NONMEM) for CL for the full data set, based on adults being 57 kg in weight and 37 years in age and children being 26 kg in weight and 10 years in age for both males and females.
) method estimates (based on one run in NONMEM) for CL for the full data set, based on adults being 57 kg in weight and 37 years in age and children being 26 kg in weight and 10 years in age for both males and females.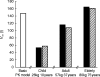
 ) method (based on one run in NONMEM) for a 26 kg, 10 year old child, a 57 kg 37 year young adult and a 80 kg 75 year old elderly subject.
) method (based on one run in NONMEM) for a 26 kg, 10 year old child, a 57 kg 37 year young adult and a 80 kg 75 year old elderly subject.
References
-
- Markham A, Sorkin EM. Ondansetron: An update of its therapeutic use in chemotherapy-induced and postoperative nausea and vomiting. Drugs. 1993;46:931–957. - PubMed
-
- Prichard JF. Ondansetron metabolism and pharmacokinetics. Seminars in Oncology. 1992;19:9–15. - PubMed
-
- Fisher V, Vickers AEM, Heitz F, et al. The polymorphic cytochrome P-4502D6 is involved in the metabolism of both 5-hydroxytryptamine antagonists, tropisetron and ondansetron. Drug Metab Dispos. 1994;22:269–274. - PubMed
-
- Dixon CM, Colthup PV, Serebjit-Singh CJ, et al. Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron. Drug Metab Dispos. 1995;23:1225–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

