Mesalazine release from a pH dependent formulation: effects of omeprazole and lactulose co-administration
- PMID: 9723828
- PMCID: PMC1873662
- DOI: 10.1046/j.1365-2125.1998.00762.x
Mesalazine release from a pH dependent formulation: effects of omeprazole and lactulose co-administration
Abstract
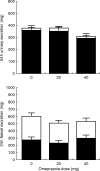
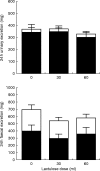
Aims: Delayed-release formulations of mesalazine often rely on the gastrointestinal luminal pH profile to deliver 5-aminosalicylic acid (5ASA) to the colon. The aim of this study was to examine the influence of luminal pH on mesalazine release.
Methods: We studied the effect of co-administration of omeprazole and lactulose on the steady-state pharmacokinetics of Eudragit S-coated mesalazine in healthy volunteers.
Results: No significant changes in urinary or faecal levels of 5ASA or its main metabolite, N-acetyl 5ASA, were apparent.
Conclusions: This study suggests that co-administration of omeprazole and lactulose does not impair the release of delayed-release mesalazine.
Figures
Similar articles
-
Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters.Br J Clin Pharmacol. 2000 Apr;49(4):323-30. doi: 10.1046/j.1365-2125.2000.00164.x. Br J Clin Pharmacol. 2000. PMID: 10759687 Free PMC article. Clinical Trial.
-
Effect of the proton pump inhibitor omeprazole on the pharmacokinetics of extended-release formulations of oxybutynin and tolterodine.J Clin Pharmacol. 2005 Aug;45(8):961-8. doi: 10.1177/0091270005278055. J Clin Pharmacol. 2005. PMID: 16027408 Clinical Trial.
-
Acid Inhibitory Effect of a Combination of Omeprazole and Sodium Bicarbonate (CDFR0209) Compared With Delayed-Release Omeprazole 40 mg Alone in Healthy Adult Male Subjects.Clin Pharmacol Drug Dev. 2018 Jan;7(1):53-58. doi: 10.1002/cpdd.331. Epub 2017 Jan 23. Clin Pharmacol Drug Dev. 2018. PMID: 28111929 Clinical Trial.
-
Clinical pharmacokinetics of slow release mesalazine.Clin Pharmacokinet. 2000 Aug;39(2):85-97. doi: 10.2165/00003088-200039020-00001. Clin Pharmacokinet. 2000. PMID: 10976656 Review.
-
Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole.Clin Pharmacokinet. 1996 Jul;31(1):9-28. doi: 10.2165/00003088-199631010-00002. Clin Pharmacokinet. 1996. PMID: 8827397 Review.
Cited by
-
Effect of acid-reducing agents on clinical relapse in ulcerative colitis with pH-dependent-released 5-aminosalicylic acid: a multicenter retrospective study in Japan.Intest Res. 2021 Apr;19(2):225-231. doi: 10.5217/ir.2020.00023. Epub 2020 Aug 18. Intest Res. 2021. PMID: 32806877 Free PMC article.
-
Drug-drug interaction profiles of proton pump inhibitors.Clin Pharmacokinet. 2010 Aug;49(8):509-33. doi: 10.2165/11531320-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20608754 Review.
References
-
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulphasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology. 1988;94:1383–1389. - PubMed
-
- Staerk Laursen L, Stockholm M, Bukhave K, Rask-Madsen J, Lauritsen K. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut. 1990;31:1271–1276. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

